
SL Paper 2
Molten sodium chloride can be electrolysed using graphite electrodes.
Draw the essential components of this electrolytic cell and identify the products that form at each electrode.
Product formed at positive electrode (anode):
Product formed at negative electrode (cathode):
State the half-equations for the oxidation and reduction processes and deduce the overall cell reaction, including state symbols.
Oxidation half-equation:
Reduction half-equation:
Overall cell reaction:
Explain why solid sodium chloride does not conduct electricity.
Markscheme
Cell showing:
container, liquid, electrodes and power supply;
No labels are required, but do not award mark if incorrect labels are used (e.g. sodium chloride solution). A line must be drawn on the container to indicate the presence of a liquid. If power supply is a battery then do not penalize electrodes incorrectly assigned as + or –.
Positive electrode (anode):
chlorine (gas) / \({\text{C}}{{\text{l}}_2}{\text{(g)}}\)
and
Negative electrode (cathode):
sodium (liquid) / Na(l);
Ignore state symbols in (i) but do not award mark for Cl.
Oxidation half-equation:
\({\text{2C}}{{\text{l}}^ - } \to {\text{C}}{{\text{l}}_2} + {\text{2}}{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{{\text{e}}^ - }\)
and
Reduction half-equation:
\({\text{N}}{{\text{a}}^ + } + {{\text{e}}^ - } \to {\text{Na}}/{\text{2N}}{{\text{a}}^ + } + {\text{2}}{{\text{e}}^ - } \to {\text{2Na}}\);
Allow e instead of \({e^ - }\).
Overall cell reaction:
\({\text{2NaCl(l)}} \to {\text{2Na(l)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}}/{\text{NaCl(l)}} \to {\text{Na(l)}} + \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}}\);
Award [1] for oxidation and reduction half-equations.
Award [1] for overall cell reaction, including correct state symbols.
Accept Na+(l) + Cl–(l) instead of NaCl(l) as a reactant.
Penalize equilibrium arrows once only.
ions not free to move when solid / ions in rigid lattice / OWTTE;
Examiners report
In part (a)(i) Some students mixed up electrolytic cells with voltaic cells and salt bridges were often seen. Others mixed up the products at the cathode and anode. For the anode, Cl was sometimes given instead of \({\text{C}}{{\text{l}}_{\text{2}}}\) meaning that the mark was not awarded. Also occasionally the electrolyte was incorrectly given as an aqueous solution.
In part (ii) The most common mistake here involved the incorrect set of state symbols. Very few candidates realised that sodium would be a liquid. Also there were equilibrium arrows incorrectly used in the redox equations.
In part (b) many candidates did not refer to ions in their answer and instead referred to the lack of delocalised electrons.
Bonds can be formed in many ways.
The landing module for the Apollo mission used rocket fuel made from a mixture of hydrazine, N2H4, and dinitrogen tetraoxide, N2O4.
N2H4(l) + N2O4(l) → 3N2(g) + 4H2O(g)
State and explain the difference in bond strength between the nitrogen atoms in a hydrazine and nitrogen molecule.
State why hydrazine has a higher boiling point than dinitrogen tetraoxide.
Determine the oxidation state of nitrogen in the two reactants.
Deduce, giving a reason, which species is the reducing agent.
Deduce the Lewis (electron dot) structures of ozone.
Markscheme
triple bond in nitrogen «molecule» AND single bond in hydrazine
triple bond stronger than single bond
OR
more shared «pairs of» electrons make bond stronger/attract nuclei more
Accept bond enthalpy values from data booklet (158 and 945 kJ\(\,\)mol–1).
[2 marks]
hydrogen bonding «between molecules, dinitrogen tetraoxide does not»
[1 mark]
N2H4: –2 AND N2O4: +4
[1 mark]
N2H4 AND oxidized/oxidation state increases
OR
N2H4 AND loses hydrogen
OR
N2H4 AND reduces/removes oxygen from N2O4
Accept “N2H4 AND gives electrons «to N2O4»”.
[1 mark]
Accept any combination of lines, dots or crosses to represent electrons.
Do not penalize missing lone pairs if already done in 3b.
Do not accept structure that represents 1.5 bonds.
[2 marks]
Examiners report
Hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}}\), releases oxygen gas, \({{\text{O}}_{\text{2}}}{\text{(g)}}\), as it decomposes according to the equation below.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
\({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of hydrogen peroxide solution was placed in a boiling tube, and a drop of liquid detergent was added to create a layer of bubbles on the top of the hydrogen peroxide solution as oxygen gas was released. The tube was placed in a water bath at 75 °C and the height of the bubble layer was measured every thirty seconds. A graph was plotted of the height of the bubble layer against time.

The experiment was repeated using solid manganese(IV) oxide, \({\text{Mn}}{{\text{O}}_{\text{2}}}{\text{(s)}}\), as a catalyst.
The decomposition of hydrogen peroxide to form water and oxygen is a redox reaction.
Explain why the curve reaches a maximum.
Use the graph to calculate the rate of decomposition of hydrogen peroxide at 120 s.
(i) Draw a curve on the graph opposite to show how the height of the bubble layer changes with time when manganese(IV) oxide is present.
(ii) Explain the effect of the catalyst on the rate of decomposition of hydrogen peroxide.
(i) Deduce the oxidation numbers of oxygen present in each of the species below.

(ii) State two half-equations for the decomposition of hydrogen peroxide.
Oxidation:
Reduction:
Markscheme
reaction is complete / all hydrogen peroxide/reactant is used up / no more bubbles are being produced / layer of bubbles is constant / OWTTE;
correctly drawn tangent to the graph at 120 s;
rate = gradient of the tangent to the graph at 120 s / \({\text{rate}} = \frac{{6.0 - 2.0}}{{240 - 0}}\);
\( = 10.017{\text{ mm}}\,{{\text{s}}^{ - 1}}\);
Accept answers in the range 0.014 to 0.020 mm\(\,\)s–1.
Units required for M3.
(i) any line which shows the height of the bubbles increasing much faster;

(ii) catalyst provides an alternative reaction pathway/mechanism with a lower activation energy;
more molecules/particles have energy greater than or equal to the activation energy / OWTTE;
Accept alternative response which refers to mechanism of heterogeneous catalysts.
(i) 
Award [2] for three correct.
Award [1] for two correct.
(ii) Oxidation:
\({{\text{H}}_2}{{\text{O}}_2} \to {{\text{O}}_{\text{2}}} + 2{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - }\);
Reduction:
\({{\text{H}}_2}{{\text{O}}_2} + {\text{2}}{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - } \to {\text{2}}{{\text{H}}_2}{\text{O}}\);
Examiners report
Part (a) was well answered.
Part (b) was either right or wrong. Few candidates drew the tangent, many being satisfied with a gradient of 4.0/120. Although a number of G2s commented on the “unusual” units of the rate (\({\text{mm}}\,{{\text{s}}^{ - 1}}\)) this did not seem to be an issue for the candidates.
In (c)(i), the line was usually correctly drawn although a significant minority drew it below the original. In (c)(ii), having stated that the catalyst provides a lower activation energy, candidates rarely explained that “more molecules/particles have energy greater than or equal to the activation energy”, many muddling the answer with that appropriate to an elevated temperature.
Most managed the oxidation numbers in (d)(i) although there were some rather curious answers for \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\). There were very few correct answers to the oxidation and reduction half equations in (ii) and this question discriminated the best candidates.
Chlorine can be made by reacting concentrated hydrochloric acid with potassium manganate(VII), \({\text{KMn}}{{\text{O}}_{\text{4}}}\).
\[{\text{2KMn}}{{\text{O}}_4}{\text{(aq)}} + {\text{16HCl(aq)}} \to {\text{2MnC}}{{\text{l}}_2}{\text{(aq)}} + {\text{2KCl(aq)}} + {\text{5C}}{{\text{l}}_2}{\text{(aq)}} + {\text{8}}{{\text{H}}_2}{\text{O(aq)}}\]
Define oxidation in terms of electron transfer.
State the oxidation number of manganese in \({\text{KMn}}{{\text{O}}_{\text{4}}}\) and in \({\text{MnC}}{{\text{l}}_{\text{2}}}\).
\({\text{KMn}}{{\text{O}}_{\text{4}}}\)
\({\text{MnC}}{{\text{l}}_{\text{2}}}\)
Deduce which species has been oxidized in this reaction and state the change in oxidation number that it has undergone.
Markscheme
Loss of (one or more) electrons;
\({\text{(KMn}}{{\text{O}}_{\text{4}}}{\text{)}} + 7\);
\({\text{(MnC}}{{\text{l}}_{\text{2}}}{\text{)}} + 2\);
Must have + sign for mark.
[1 max] if roman numerals or 7+ or 2+ used or if + signs are missing.
\({\text{C}}{{\text{l}}^ - }\) / chloride / chlorine / Cl (has been oxidized) / HCl;
oxidation number from –1 to 0 / has increased by one;
If HCl is given for first mark, it must be clear that it is the Cl that has the change of oxidation number.
Examiners report
This question was probably the best answered on the paper. Most candidates were able to score the mark for the definition of oxidation.
In Part (b)(i) many candidates scored both marks, though a significant number received a single mark penalty for not including the + sign.
In (b)(ii) most candidates were able to identify chlorine as the substance oxidised but many suggested rather odd values for its oxidation states.
Phosphine (IUPAC name phosphane) is a hydride of phosphorus, with the formula PH3.
(i) Draw a Lewis (electron dot) structure of phosphine.
(ii) Outline whether you expect the bonds in phosphine to be polar or non-polar, giving a brief reason.
(iii) Explain why the phosphine molecule is not planar.
(iv) Phosphine has a much greater molar mass than ammonia. Explain why phosphine has a significantly lower boiling point than ammonia.
Phosphine is usually prepared by heating white phosphorus, one of the allotropes of phosphorus, with concentrated aqueous sodium hydroxide. The equation for the reaction is:
P4 (s) + 3OH− (aq) + 3H2O (l) → PH3 (g) + 3H2PO2− (aq)
(i) Identify one other element that has allotropes and list two of its allotropes.
Element:
Allotrope 1:
Allotrope 2:
(ii) The first reagent is written as P4, not 4P. Describe the difference between P4 and 4P.
(iii) The ion H2PO2− is amphiprotic. Outline what is meant by amphiprotic, giving the formulas of both species it is converted to when it behaves in this manner.
(iv) State the oxidation state of phosphorus in P4 and H2PO2−.
P4:
H2PO2−:
(v) Oxidation is now defined in terms of change of oxidation number. Explore how earlier definitions of oxidation and reduction may have led to conflicting answers for the conversion of P4 to H2PO2− and the way in which the use of oxidation numbers has resolved this.
2.478 g of white phosphorus was used to make phosphine according to the equation:
P4(s) +3OH−(aq)+3H2O(l) → PH3(g)+3H2PO2−(aq)
(i) Calculate the amount, in mol, of white phosphorus used.
(ii) This phosphorus was reacted with 100.0 cm3 of 5.00 mol dm−3 aqueous sodium hydroxide. Deduce, showing your working, which was the limiting reagent.
(iii) Determine the excess amount, in mol, of the other reagent.
(iv) Determine the volume of phosphine, measured in cm3 at standard temperature and pressure, that was produced.
Markscheme
(i)
Accept structures using dots and/or crosses to indicate bonds and/or lone pair.
(ii)
non-polar AND P and H have the same electronegativity
Accept “similar electronegativities”.
Accept “polar” if there is a reference to a small difference in electronegativity and apply ECF in 1 a (iv).
(iii)
4 electron domains/pairs/negative charge centres «around the central atom»
OR
a lone/non-bonding pair «and three bonding pairs around the central atom»
repulsion between electron domains/pairs/negative charge centres «produces non-planar shape»
OR
«repulsion causes» tetrahedral orientation/pyramidal shape
(iv)
PH3 has London «dispersion» forces
NH3 forms H-bonds
H-bonds are stronger
OR
London forces are weaker
Accept van der Waals’ forces, dispersion forces and instantaneous dipole – induced dipole forces.
Accept “dipole-dipole forces” as molecule is polar.
H-bonds in NH3 (only) must be mentioned to score [2].
Do not award M2 or M3 if:
• implies covalent bond is the H-bond
• implies covalent bonds break.
Accept “dipole-dipole forces are weaker”.
(i)
Element
carbon/C
OR
oxygen/O/O2
Allotropes
Award [1] for two of:
diamond
graphite
graphene
C60 / buckminsterfullerene
OR
ozone/O3 AND «diatomic/molecular» oxygen/O2
Accept two correctly named allotropes of any other named element (S, Se, Sn, As, etc.).
Accept fullerene, “buckyballs” etc. instead of buckminsterfullerene.
(ii)
P4 is a molecule «comprising 4P atoms» AND 4P is four/separate «P» atoms
OR
P4 represents «4P» atoms bonded together AND 4P represents «4» separate/non-bonded «P» atoms
(iii)
can act as both a «Brønsted–Lowry» acid and a «Brønsted–Lowry» base
OR
can accept and/or donate a hydrogen ion/proton/H+
HPO22− AND H3PO2
(iv)H2PO2− : +1
OR
negative charge «on product/H2PO2− » /gain of electrons so could be reduction
Do not award M3 for “oxidation number changes”.
(i)
«\(\left\langle {\frac{{2.478}}{{4 \times 30.97}}} \right\rangle \)»= 0.02000«mol»
(ii)
n(NaOH)=«0.1000×5.00=»0.500«mol» AND P4/phosphorus is limiting reagent
Accept n(H2O) =\(\frac{{100}}{{18}}\) = 5.50 AND P4 is limiting reagent.
(iii)
amount in excess «= 0.500 - (3 × 0.02000)» = 0.440 «mol»
(iv)
«22.7 × 1000 × 0.02000» = 454 «cm3»
Accept methods employing pV = nRT, with p as either 100 (454 cm3) or 101.3 kPa (448 cm3).
Do not accept answers in dm3.
Examiners report
Consider the following equilibrium.
\[\begin{array}{*{20}{l}} {{\text{2S}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_3}{\text{(g)}}}&{\Delta {H^\Theta } = - 198{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
State and explain the effect of increasing the temperature on the yield of sulfur trioxide.
State the effect of a catalyst on the value of \({K_{\text{c}}}\).
State and explain the effect of a catalyst on the position of equilibrium.
Define oxidation in terms of oxidation numbers.
Describe using a labelled diagram, the essential components of an electrolytic cell.
Explain why solid sodium chloride does not conduct electricity but molten sodium chloride does.
Molten sodium chloride undergoes electrolysis in an electrolytic cell. For each electrode deduce the half-equation and state whether oxidation or reduction takes place. Deduce the equation of the overall cell reaction including state symbols.
Electrolysis has made it possible to obtain reactive metals such as aluminium from their ores, which has resulted in significant developments in engineering and technology. State one reason why aluminium is preferred to iron in many uses.
Outline two differences between an electrolytic cell and a voltaic cell.
Markscheme
\({\text{(}}{K_{\text{c}}} = {\text{)[S}}{{\text{O}}_{\text{3}}}{{\text{]}}^{\text{2}}}{\text{/[}}{{\text{O}}_{\text{2}}}{\text{][S}}{{\text{O}}_{\text{2}}}{{\text{]}}^{\text{2}}}\);
yield (of \({\text{S}}{{\text{O}}_{\text{3}}}\)) decreases;
forward reaction is exothermic / reverse/backwards reaction is endothermic / equilibrium shifts to absorb (some of) the heat;
Do not accept exothermic reaction or Le Châtelier’s Principle.
Do not allow ECF.
no effect;
no effect;
the rates of both the forward and reverse reactions increase equally;
increase in the oxidation number;
Annotated diagram of cell showing:
power supply/battery;
electrolyte;
cathode/negative electrode and anode/positive electrode;
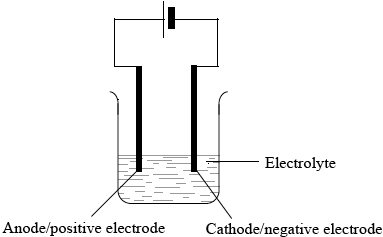
(solid) ions in a lattice / ions cannot move;
(molten) ions mobile / ions free to move;
reduction occurs at the cathode/negative electrode and oxidation occurs at the anode/positive electrode;
Cathode/negative electrode: \({\text{N}}{{\text{a}}^ + } + {{\text{e}}^ - } \to {\text{Na}}\);
Anode/positive electrode: \({\text{2C}}{{\text{l}}^ - } \to {\text{C}}{{\text{l}}_2} + {\text{2}}{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2} + {{\text{e}}^ - }\);
Award [1 max] if the two electrodes are not labelled/labelled incorrectly for the two half-equations.
Overall cell reaction: \({\text{N}}{{\text{a}}^ + }{\text{(1)}} + {\text{C}}{{\text{l}}^ - }{\text{(1)}} \to {\text{Na(1)}} + \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}}\)
Award [1] for correct equation and [1] for correct state symbols.
Allow NaCl(l) instead of Na+(l) and Cl–(l).
Al does not corrode/rust / Al is less dense/better conductor/more malleable;
Accept Al is a lighter (metal compared to Fe).
Accept converse argument.
electrolytic cell converts electrical energy to chemical energy and voltaic cell converts chemical energy to electrical energy / electrolytic cell uses electricity to carry out a (redox) chemical reaction and voltaic cell uses a (redox) chemical reaction to produce electricity / electrolytic cell requires a power supply and voltaic cell does not;
electrolytic cell involves a non-spontaneous (redox) reaction and voltaic cell involves a spontaneous (redox) reaction;
in an electrolytic cell, cathode is negative and anode is positive and vice-versa for a voltaic cell / electrolytic cell, anode is positive and voltaic cell, anode is negative / electrolytic cell, cathode is negative and voltaic cell, cathode is positive;
voltaic cell has two separate solutions and electrolytic cell has one solution / voltaic cell has salt bridge and electrolytic cell has no salt bridge;
electrolytic cell, oxidation occurs at the positive electrode/anode and voltaic cell, oxidation occurs at the negative electrode/anode and vice-versa;
Examiners report
Nearly all candidates deduced the equilibrium constant expression for the reaction given in (a) (i).
there were many good and complete answers here for (a) (ii). Some candidates did not state that the forward reaction was exothermic or the reverse reaction was endothermic, when trying to decide the effect of an increase in temperature on the yield of \({\text{S}}{{\text{O}}_{\text{3}}}\).
In (a) (iii) most candidates correctly stated that the catalyst would not have any effect on the value of \({K_{\text{c}}}\).
In part (iv) many candidates correctly stated that the catalyst would not have any effect on the position of equilibrium, but some did not explain why.
In (b) (i) some candidates defined oxidation as the loss of electrons but not in terms of oxidation numbers, as required by the question.
Some candidates described a voltaic cell instead of an electrolytic cell in (b) (ii). In some cases the electrodes were wrongly labelled or wrongly connected to the battery and the electrolyte was missing.
A large number of candidates stated that solid sodium chloride did not conduct electricity because it did not contain electrons in (iii). However some gave the correct answer indicating the free/moving ions as the particles responsible for the conductivity.
Part (b) (iv) was generally well answered. Most candidates lost a mark because they did not give the correct state symbols in the overall reaction.
Most candidates gave a correct answer as to why aluminium is preferred to iron in many uses in (b) (v).
There were very good answers indicating the main differences between an electrolytic cell and a voltaic cell in (vi).
The element antimony, Sb, is usually found in nature as its sulfide ore, stibnite, \({\text{S}}{{\text{b}}_{\text{2}}}{{\text{S}}_{\text{3}}}\). This ore was used two thousand years ago by ancient Egyptian women as a cosmetic to darken their eyes and eyelashes.
One method of extracting antimony from its sulfide ore is to roast the stibnite in air. This forms antimony oxide and sulfur dioxide. The antimony oxide is then reduced by carbon to form the free element.
Deduce the oxidation number of antimony in stibnite.
Deduce one other common oxidation number exhibited by antimony in some of its compounds.
Deduce the chemical equations for these two reactions.
Markscheme
+3;
Do not accept 3, 3+ or the use of Roman numerals.
+5 / –3;
Penalize incorrect format only if not penalized in (a)(i).
\({\text{S}}{{\text{b}}_2}{{\text{S}}_3} + {\text{4}}\frac{1}{2}{{\text{O}}_2} \to {\text{S}}{{\text{b}}_2}{{\text{O}}_3} + {\text{3S}}{{\text{O}}_2}/{\text{2S}}{{\text{b}}_2}{{\text{S}}_3} + {\text{9}}{{\text{O}}_2} \to {\text{2S}}{{\text{b}}_2}{{\text{O}}_3} + {\text{6S}}{{\text{O}}_2}\);
\({\text{2S}}{{\text{b}}_2}{{\text{O}}_3} + {\text{3C}} \to {\text{4Sb}} + {\text{3C}}{{\text{O}}_2}/{\text{S}}{{\text{b}}_2}{{\text{O}}_3} + {\text{3C}} \to {\text{2Sb}} + {\text{3CO}}\);
Ignore state symbols.
Examiners report
This question proved difficult to candidates as the antimony was unfamiliar to them. However they were expected to just apply what they already knew about other members of the group such as nitrogen and phosphorous. Those that could calculate the oxidation state of antinomy in stibnite often forgot to add the + charge.
Writing the chemical equations proved difficult for candidates but again many picked up 1 out of 2 marks as ecf was applied.
Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is a weak base.
Iron is more reactive than copper.
Draw the Lewis structure of ammonia and state the shape of the molecule and its bond angles.
The conjugate acid of ammonia is the ammonium ion, \({\text{NH}}_4^ + \). Draw the Lewis structure of the ammonium ion and deduce its shape and bond angles.
Describe two different properties that could be used to distinguish between a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a strong monoprotic acid and a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a weak monoprotic acid.
Explain, using the Brønsted-Lowry theory, how water can act either as an acid or a base. In each case identify the conjugate acid or base formed.
Draw a labelled diagram of a voltaic cell made from an \({\text{Fe(s)}}/{\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) half-cell connected to a \({\text{Cu(s)}}/{\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) half-cell. In your diagram identify the positive electrode (cathode), the negative electrode (anode) and the direction of electron flow in the external circuit.
Deduce the half-equations for the reactions taking place at the positive electrode (cathode) and negative electrode (anode) of this voltaic cell.
Deduce the overall equation for the reaction taking place in the voltaic cell and determine which species acts as the oxidizing agent and which species has been reduced.
Markscheme
 ;
;
Accept any combination of dots/crosses and lines to represent electron pairs.
(trigonal/triangular) pyramid;
Allow 3D representation using wedges and dotted bonds of trigonal pyramidal molecule.
107°;
Accept any angle between 105° and 108.5°.
No ECF for shape based on incorrect Lewis structure.
 ;
;
Charge needed for mark.
Allow a 3D representation using wedges and dotted bonds of tetrahedral molecule.
109.5°/109°/109° 28';
No ECF for shape based on incorrect Lewis structure.
(measuring) the pH / the strong acid solution will have a lower pH;
conductivity (measurement) / the strong acid will be a better conductor;
the strong acid will react more vigorously with metals/carbonates / the reaction with metals/carbonates;
the heat change when it is neutralized with a base will be different / heat of neutralization / OWTTE;
water can act as a Brønsted-Lowry acid by donating a proton/\({{\text{H}}^ + }\) to form \({\text{O}}{{\text{H}}^ - }\);
water can act as a Brønsted-Lowry base by accepting a proton/\({{\text{H}}^ + }\) to form \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\);
Accept equations showing the above clearly labelling the acid and basic behaviour and the conjugate acid or base.
Award [1 max] for correct definition of how water can act as a Brønsted-Lowry acid or base.

correct diagram including voltmeter/meter, 4 correct species (state symbols not required) and connecting wires;
No credit if wires to electrodes immersed in the solutions.
labelled salt bridge;
Do not accept name of salt (e.g. potassium nitrate) in place of salt bridge.
correctly labelled electrodes (+)/cathode and (–)/anode;
flow of electrons from Fe to Cu in external circuit;
positive electrode: \({\text{C}}{{\text{u}}^{2 + }} + {\text{2}}{{\text{e}}^ - } \to {\text{Cu}}\);
negative electrode: \({\text{Fe}} \to {\text{F}}{{\text{e}}^{2 + }} + {\text{2}}{{\text{e}}^ - }\);
Award [1] if equations correct but at wrong electrodes or if electrodes are missing.
Award [2] for correct equations if electrodes are missing but were correctly labelled in diagram.
Accept e instead of \({e^ - }\).
Ignore state symbols.
Penalize \( \rightleftharpoons \) once only in equations in (ii) and (iii).
\({\text{Fe}} + {\text{C}}{{\text{u}}^{2 + }} \to {\text{F}}{{\text{e}}^{2 + }} + {\text{Cu}}\);
Ignore state symbols.
\({\text{C}}{{\text{u}}^{2 + }}\) is the oxidizing agent and the species that is reduced;
Examiners report
Candidates could draw the Lewis structures in part (a) and generally they could name the shape and suggest the bond angle.
Most knew what a Lewis acid was but some were careless in their definition and said it was an electron acceptor instead of an electron pair acceptor.
Generally candidates could suggest ways of distinguishing between strong and weak acids using pH or conductivity.
The final part of this question caused some difficulty though as students found it hard to show water acting as an acid and a base even though many could correctly state that an acid is a proton donor and a base is a proton acceptor.
Part (b) focused on electrochemistry and although some candidates were able to score 4 marks most lost marks for their diagrams which were often incomplete and/or incorrectly annotated.
Students that could draw the diagram had little problem writing the equations, however many could not do them correctly.
Students that could draw the diagram had little problem writing the equations, however many could not do them correctly. This carried through to the final part of the question and those that could write the half equations could generally write the overall equation. Identifying the oxidizing agent and the species that has been reduced proved tricky as students were reluctant to suggest the same species- \({\text{C}}{{\text{u}}^{2 + }}\), also some students just said copper which was not specific enough to gain the mark.
Sodium oxide, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\), is a white solid with a high melting point.
Explain why solid sodium oxide is a non-conductor of electricity.
Molten sodium oxide is a good conductor of electricity. State the half-equation for the reaction occurring at the positive electrode during the electrolysis of molten sodium oxide.
State the acid-base nature of sodium oxide.
State the equation for the reaction of sodium oxide with water.
Markscheme
in the solid state ions are in fixed positions/there are no moveable ions / OWTTE;
Do not accept answer that refers to atoms or molecules.
\({\text{2}}{{\text{O}}^{2 - }} \to {{\text{O}}_2} + {\text{4}}{{\text{e}}^ - }/{{\text{O}}^{2 - }} \to \frac{1}{2}{{\text{O}}_2} + {\text{2}}{{\text{e}}^ - }\);
Accept e instead of e–.
basic;
Allow alkaline
\({\text{N}}{{\text{a}}_2}{\text{O}} + {{\text{H}}_2}{\text{O}} \to {\text{2NaOH}}/{\text{N}}{{\text{a}}_2}{\text{O}} + {{\text{H}}_2}{\text{O}} \to {\text{2N}}{{\text{a}}^ + } + {\text{2O}}{{\text{H}}^ - }\);
Do not accept \( \rightleftharpoons \)
Examiners report
This was expected to be a high-scoring question but this was not found in practice. In Part (a) there were many references to delocalised/mobile electrons and also molecules and atoms. It did not appear that the structural properties of ionic substances are well understood.
There were many attempts in (b) which involved the sodium ion rather than the oxide and those who chose oxide often had difficulty in producing a balanced equation.
The best answered part of this question was Part (c) though a significant percentage described it as a weak base.
The best answered part of this question was Part (c) though a significant percentage described it as a weak base.
The data below is from an experiment used to determine the percentage of iron present in a sample of iron ore. This sample was dissolved in acid and all of the iron was converted to \({\text{F}}{{\text{e}}^{{\text{2 + }}}}\). The resulting solution was titrated with a standard solution of potassium manganate(VII), \({\text{KMn}}{{\text{O}}_{\text{4}}}\). This procedure was carried out three times. In acidic solution, \({\text{MnO}}_4^ - \) reacts with \({\text{F}}{{\text{e}}^{2 + }}\) ions to form \({\text{M}}{{\text{n}}^{2 + }}\) and \({\text{F}}{{\text{e}}^{3 + }}\) and the end point is indicated by a slight pink colour.


Deduce the balanced redox equation for this reaction in acidic solution.
Identify the reducing agent in the reaction.
Calculate the amount, in moles, of \({\text{MnO}}_4^ - \) used in the titration.
Calculate the amount, in moles, of Fe present in the \(3.682 \times {10^{ - 1}}{\text{ g}}\) sample of iron ore.
Determine the percentage by mass of Fe present in the \(3.682 \times {10^{ - 1}}{\text{ g}}\) sample of iron ore.
Markscheme
\[{\text{MnO}}_4^ - {\text{(aq)}} + {\text{5F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{5F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
Award [2] if correctly balanced.
Award [1] for correctly placing H+ and H2O.
Award [1 max] for correct balanced equation but with electrons shown.
Ignore state symbols.
\({\text{F}}{{\text{e}}^{2 + }}\) / iron(II);
Do not accept iron.
\({\text{n}} = 2.152 \times {10^{ - 2}} \times 2.250 \times {10^{ - 2}}\);
\(4.842 \times {10^{ - 4}}{\text{ (mol)}}\);
Award [1] for correct volume
Award [1] for correct calculation.
1 mol of \({\text{MnO}}_4^ - \) reacts with 5 mol of \({\text{F}}{{\text{e}}^{2 + }}\);
\(5 \times 4.842 \times {10^{ - 4}} = 2.421 \times {10^{ - 3}}{\text{ (mol)}}\);
(same number of moles of Fe in the iron ore)
Allow ECF from part (a) and (c) provided some mention of mole ratio is stated.
\(2.421 \times {10^{ - 3}} \times 55.85 = 0.1352{\text{ (g)}}\);
\(\frac{{0.1352}}{{0.3682}} \times 100 = 36.72\% \);
Allow ECF from part (d).
Examiners report
Most G2 comments on Section A were about this question. Many commented that titration is not part of the SL syllabus; however it is the expectation that students would cover this and other basic chemical techniques as part of their practical programme. Question 1 in all papers is meant to be data response and students will be expected to be familiar with experimental techniques. Also, there was some confusion caused because there was one sample and three titres. However this unfortunate cause of confusion did not seem to impact on candidate performance as poor performance was found throughout the question even with some very routine questions. Generally this question was poorly answered. In a) not many candidates managed to write the correct balanced equation, however many gave the correct species that were missing, \({{\text{H}}^ + }\) and \({{\text{H}}_{\text{2}}}{\text{O}}\).
Most candidates were able to identify the reducing agent although a few candidates just mentioned “iron” or Fe, but metallic iron was not in the equation.
In (c) candidates were not familiar with the process of selecting the 2 best titres and averaging them. Some chose the first written, some averaged all three and some weaker candidates merely added the 3 titres and used this. Some candidates also forgot to convert cm3 to dm3.
In 1(e), a few candidates scored ECF marks for the % based on n(Fe) calculated in (d). A couple of candidates realised that their answer to (d) did not help and followed on from (c) to find the number of moles of \({\text{F}}{{\text{e}}^{2 + }}\) and hence the % of Fe, scoring ECF marks.
Vanadium, another transition metal, has a number of different oxidation states.
Determine the oxidation state of vanadium in each of the following species.
Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to form V3+(aq).
Markscheme
V2O5: +5
VO2+: +4
Do not penalize incorrect notation twice.
[2 marks]
VO2+(aq) + V2+(aq) + 2H+(aq) → 2V3+(aq) + H2O(l)
Accept equilibrium sign.
[1 mark]
Examiners report
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) \( \rightleftharpoons \) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) \( \rightleftharpoons \) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
Determine the average oxidation state of carbon in ethene and in ethane-1,2-diol.
Ethene:
Ethane-1,2-diol:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
Ethane-1,2-diol can be oxidized first to ethanedioic acid, (COOH)2, and then to carbon dioxide and water. Suggest the reagents to oxidize ethane-1,2-diol.
Markscheme
(i)
\(\ll {K_{\text{C}}} = \gg \frac{{\left[ {{\text{HOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}} \right]}}{{{{\left[ {{\text{CO}}} \right]}^{\text{2}}} \times {{\left[ {{{\text{H}}_{\text{2}}}} \right]}^{\text{3}}}}}\)
(ii)
Position of equilibrium: moves to right OR favours product
Kc: no change OR is a constant at constant temperature
(iii)
Bonds broken: 2C≡O + 3(H-H) / 2(1077kJmol-1) + 3(436kJmol-1) / 3462 «kJ»
Bonds formed: 2(C-O) + 2(O-H) + 4(C-H) + (C-C) / 2(358kJmol-1) + 2(463kJmol-1) + 4(414kJmol-1) + 346kJmol-1 / 3644 «kJ»
«Enthalpy change = bonds broken - bonds formed = 3462 kJ - 3644 kJ =» -182 «kJ»
Award [3] for correct final answer.
Award [2 max] for «+»182 «kJ».
(iv)
in (a)(iii) gas is formed and in (a)(iv) liquid is formed
OR
products are in different states
OR
conversion of gas to liquid is exothermic
OR
conversion of liquid to gas is endothermic
OR
enthalpy of vapourisation needs to be taken into account
Accept product is «now» a liquid.
Accept answers referring to bond enthalpies being means/averages.
Ethene: –2
Ethane-1,2-diol: –1
Do not accept 2–, 1– respectively.
ethane-1,2-diol can hydrogen bond to other molecules «and ethene cannot»
OR
ethane-1,2-diol has «significantly» greater van der Waals forces
Accept converse arguments.
Award [0] if answer implies covalent bonds are broken
hydrogen bonding is «significantly» stronger than other intermolecular forces
acidified «potassium» dichromate«(VI)»/H+ AND K2Cr2O7/H+ AND Cr2O72-
OR
«acidified potassium» manganate(VII)/ «H+» KMnO4 /«H+» MnO4-
Accept Accept H2SO4 or H3PO4 for H+.
Accept “permanganate” for “manganate(VII)”.
Examiners report
Brass is a copper containing alloy with many uses. An analysis is carried out to determine the percentage of copper present in three identical samples of brass. The reactions involved in this analysis are shown below.
\[\begin{array}{*{20}{l}} {{\text{Step 1: Cu(s)}} + {\text{2HN}}{{\text{O}}_3}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2N}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}} \\ {{\text{Step 2: 4}}{{\text{I}}^ - }{\text{(aq)}} + {\text{2C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{2CuI(s)}} + {{\text{I}}_2}{\text{(aq)}}} \\ {{\text{Step 3: }}{{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} \to {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}}} \end{array}\]
(a) (i) Deduce the change in the oxidation numbers of copper and nitrogen in step 1.
Copper:
Nitrogen:
(ii) Identify the oxidizing agent in step 1.
(b) A student carried out this experiment three times, with three identical small brass nails, and obtained the following results.
\[{\text{Mass of brass}} = 0.456{\text{ g}} \pm 0.001{\text{ g}}\]

(i) Calculate the average amount, in mol, of \({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\) added in step 3.
(ii) Calculate the amount, in mol, of copper present in the brass.
(iii) Calculate the mass of copper in the brass.
(iv) Calculate the percentage by mass of copper in the brass.
(v) The manufacturers claim that the sample of brass contains 44.2% copper by mass. Determine the percentage error in the result.
(c) With reference to its metallic structure, describe how brass conducts electricity.
Markscheme
(a) (i) Copper:
0 to +2 / increases by 2 / +2 / 2+;
Allow zero/nought for 0.
Nitrogen:
+5 to +4 / decreases by 1 / –1 / 1–;
Penalize missing + sign or incorrect notation such as 2+, 2+ or II, once only.
(ii) nitric acid/\({\text{HN}}{{\text{O}}_{\text{3}}}\) / \({\text{NO}}_3^ - \)/nitrate;
Allow nitrogen from nitric acid/nitrate but not just nitrogen.
(b) (i) \(0.100 \times 0.0285\);
\(2.85 \times {10^{ - 3}}{\text{ (mol)}}\);
Award [2] for correct final answer.
(ii) \(2.85 \times {10^{ - 3}}{\text{ (mol)}}\);
(iii) \(63.55 \times 2.85 \times {10^{ - 3}} = 0.181{\text{ g}}\);
Allow 63.5.
(iv) \(\left( {\frac{{0.181}}{{0.456}} \times 100 = } \right){\text{ }}39.7\% \);
(v) \(\left( {\frac{{44.2 - 39.7}}{{44.2}} \times 100 = } \right){\text{ }}10.2\% \);
Allow 11.3% i.e. percentage obtained in (iv) is used to divide instead of 44.2%.
(c) Brass has:
delocalized electrons / sea of mobile electrons / sea of electrons free to move;
No mark for just “mobile electrons”.
Examiners report
There were several G2 comments on this question, all of which claimed that the question was difficult for SL candidates especially as a three-step reaction process was involved. Certainly some of the weaker candidates struggled with this question, but with the application of ECF marks, most candidates should have been able to score the majority of marks in the question. What was more worrying was the large number of candidates who scored zero or close to zero marks on Q.1, which meant they had little idea of a titration from their exposure to laboratory work in the programme as a whole.
In (a) (i), most candidates showed a reasonable understanding of oxidation numbers, but relatively few scored full marks as they did not read the question which asked explicitly for the change in oxidation numbers. A number also incorrectly wrote 5+ going to 4+ instead of +5 going to +4 i.e. they mixed up charges with oxidation numbers. In the oxidizing agent question in part (ii), the most common mistake was candidates writing nitrogen, instead of the nitric acid, which is the agent involved. In (b), candidates typically either did very well or scored almost no marks at all. In (i), a number of candidates did not convert to dm3 and some did not use the average volume in their calculations, again failing to read the question carefully. (c) however was well answered, though some candidates made reference to the ions as charge carriers rather than giving a description of delocalized electrons. Other candidates stated just mobile electrons instead of stating sea of mobile electrons which was required for the mark.
Consider the following three redox reactions.
\[\begin{array}{*{20}{l}} {{\text{Cd(s)}} + {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} \to {\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{Ni(s)}}} \\ {{\text{Ni(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \to {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}} \\ {{\text{Zn(s)}} + {\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} \to {\text{Z}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{Cd(s)}}} \end{array}\]
(i) Draw an annotated diagram of a voltaic cell composed of a magnesium electrode in \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) magnesium nitrate solution and a silver electrode in \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) silver nitrate solution. State the direction of electron flow on your diagram.
(ii) Deduce half-equations for the oxidation and reduction reactions.
(i) Deduce the order of reactivity of the four metals, cadmium, nickel, silver and zinc and list in order of decreasing reactivity.
(ii) Identify the best oxidizing agent and the best reducing agent.
(i) Solid sodium chloride does not conduct electricity but molten sodium chloride does. Explain this difference.
(ii) Outline what happens in an electrolytic cell during the electrolysis of molten sodium chloride using inert electrodes. Deduce equations for the reactions occurring at each electrode.
(i) A state of equilibrium can exist when a piece of copper metal is placed in a solution of copper(II) sulfate. Outline the characteristics of a chemical system in dynamic equilibrium.
(ii) For an exothermic reaction state how an increase in temperature would affect both \({K_{\text{c}}}\) and the position of equilibrium.
Markscheme
(i) 
correctly labelled electrodes and solutions;
labelled salt bridge;
voltmeter;
Allow bulb or ammeter.
direction of electron flow;
(ii) Oxidation:
\({\text{Mg(s)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
Reduction:
\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to {\text{Ag(s)}}\);
Ignore state symbols.
Award [1 max] if equations not labelled reduction or oxidation or labelled the wrong way round.
Allow e instead of e–.
Penalize equilibrium sign or reversible arrows once only in parts (a) (ii) and (c) (ii).
(i) \({\text{Zn}} > {\text{Cd}} > {\text{Ni}} > {\text{Ag}}\)
Zn most reactive;
rest of order correct;
(ii) Best oxidizing agent:
\({\text{A}}{{\text{g}}^ + }\);
Do not accept Ag.
Best reducing agent:
Zn;
Do not accept Zn2+.
(i) sodium chloride crystals consist of ions in a (rigid) lattice / ions cannot move (to electrodes) / OWTTE;
when melted ions free to move / ions move when potential difference/voltage applied;
(ii) positive sodium ions/\({\text{N}}{{\text{a}}^ + }\) move to negative electrode/cathode and negative chloride ions/ \({\text{C}}{{\text{l}}^ - }\) move to positive electrode/anode;
electrons released to positive electrode/anode by negative ions and accepted from negative electrode/cathode by positive ions / reduction occurs at the negative electrode/cathode and oxidation occurs at the positive electrode/anode / \({\text{N}}{{\text{a}}^ + }\) ions are reduced and \({\text{C}}{{\text{l}}^ - }\) ions are oxidized;
(Positive electrode/anode):
\({\text{2C}}{{\text{l}}^ - } \to {\text{C}}{{\text{l}}_2} + {\text{2}}{{\text{e}}^ - }{\text{ / C}}{{\text{l}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2} + {{\text{e}}^ - }\);
(Negative electrode/cathode):
\({\text{2N}}{{\text{a}}^ + } + {\text{2}}{{\text{e}}^ - } \to {\text{2Na / N}}{{\text{a}}^ + } + {{\text{e}}^ - } \to {\text{Na}}\);
Award [1 max] if equations not labelled or labelled wrong way round.
Allow e instead of e–.
Penalize equilibrium sign or reversible arrows once only in parts (a) (ii) and (c) (ii).
(i) macroscopic properties remain constant / concentrations remain constant / no change to copper solution seen;
rate of reverse/backwards reaction = rate of forward reaction;
(ii) \({K_{\text{c}}}\) decreases;
position of equilibrium shifts to left;
Examiners report
This question was also chosen by approximately 40% of candidates. In (a), the diagram was reasonably attempted by most candidates, with just a few candidates giving both electrodes in one beaker. Some candidates omitted to include a voltmeter and other common mistakes included omission of states and incorrect direction of electron flow. In (ii), fewer candidates scored these marks and many equations were not labelled explicitly as oxidation and reduction. Other common errors included incorrect charges for the silver and magnesium ions.
In (b), most candidates were able to place the metals in order, though a small minority misread the question, and gave zinc as the least reactive. In (ii), zinc was generally given as the best reducing agent, but often silver metal rather than silver(I) ion as given as the best oxidizing agent.
There were many references to sodium chloride having a metallic structure in (c) (i), and describing its conduction in terms of electrons rather than ions. In (ii), very few candidates mentioned that the ions move towards the oppositely charged electrode. The nature of the electrolytic process was not well explained.
The characteristics of a chemical system in a dynamic equilibrium in (d) (i) typically were understood by most candidates, although many just scored one mark. (ii) was well answered.
Phosphorus tribromide (\({\text{PB}}{{\text{r}}_{\text{3}}}\)) is used to manufacture alprazolam, a drug used to treat anxiety disorders. Methanal (HCHO) is used as a disinfectant.
Consider the following reaction sequence:

Deduce the balanced chemical equation for the reaction between sodium and sulfur. State the electron arrangements of the reactants and product, and explain whether sulfur is oxidized or reduced.
Describe the acid-base character of the oxides of the period 3 elements, Na to Cl. For the compounds sodium oxide and phosphorus(V) oxide, state the balanced chemical equations for the reaction of each oxide with water.
For each of the species \({\text{PB}}{{\text{r}}_{\text{3}}}\) and HCHO:
• deduce the Lewis structure.
• predict the shape and bond angle.
Explain why \({\text{PB}}{{\text{r}}_{\text{3}}}\) is a polar molecule.
State the name of A.
Describe the redox behaviour of chromium with reference to oxidation numbers in the conversion of B to C.
Define the term oxidizing agent and identify the oxidizing agent in the following
reaction.
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {{\text{I}}^ - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{IO}}_3^ - {\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
Markscheme
\({\text{2Na(s)}} + {\text{S(s)}} \to {\text{N}}{{\text{a}}_2}{\text{S(s)}}/{\text{2Na(s)}} + \frac{1}{2}{{\text{S}}_2}{\text{(g)}} \to {\text{N}}{{\text{a}}_2}{\text{S(s)}}/{\text{16Na(s)}} + {{\text{S}}_2}{\text{(s)}} \to {\text{8N}}{{\text{a}}_2}{\text{S(s)}}\);
Ignore state symbols.
Na: 2, 8,1 and S: 2, 8, 6;
\({\text{N}}{{\text{a}}^ + }\): 2, 8 and \({{\text{S}}^{2 - }}\): 2,8,8;
reduced since it has gained electrons / reduced since oxidation number has decreased;
Do not award mark if incorrect oxidation numbers are given.
Na, Mg: basic
Al: amphoteric
Do not accept amphiprotic.
Si to Cl: acidic
Award [2] for all three listed sets correct, [1] for one or two listed sets correct.
Award [1] for stating oxides become more basic towards left/Na and more acidic towards right/Cl.
Do not penalize incorrect formulas of oxides.
\({\text{N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
Allow P2O3(s) \( + \) 3H2O(l) \( \to \) 2H3PO4(aq).

Do not allow ECF in this question from incorrect Lewis structure.
Br more electronegative than P / P–Br bond polar;
bond dipoles do not cancel / there is a net dipole / asymmetric distribution of electron cloud;

Allow polar bonds do not cancel or that it is an asymmetric molecule.
Award [2] for diagram showing net dipole moment as shown.
chromium(III) oxide;
Do not award mark for chromium oxide.
chromium is neither oxidized or reduced since there is no change in oxidation number/+6 to +6;
substance reduced / causes other substance to be oxidized / increase oxidation number of another species / gains electrons / OWTTE;
Oxidizing agent:
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }\) / dichromate (ion);
Examiners report
This was one of the least popular questions but those candidates that did attempt it, often performed well. In part (a) the equation was well answered, as were the electron arrangements of sodium and sulfur, but candidates struggled with the electron arrangements of the ions. Also, some forgot to give a reason as to why sulfur is reduced.
The first part on acid-base behaviour was well answered though a few stated that silicon is amphoteric which is incorrect, unfortunately this is an error that has appeared in some IB textbooks. As regards the equations, hydrogen was often given as a product, and although many could successfully write the equation of sodium oxide with water, very few could successfully write the equation with phosphorous (V) oxide.
In part (c) candidates could draw the Lewis structures and generally they could name the shape and suggest the bond angle. However lone pairs were often omitted, especially on oxygen and bromine.
Explaining molecular polarity often was more challenging, and clearly it is poorly understood.
Few candidates correctly used the Roman numeral III.
Many candidates did realise in part (ii) that there was no change in oxidation number of chromium and so no redox reaction.
In part (iii) candidates could define what an oxidizing agent was and most correctly identified dichromate, as the oxidizing agent, however some just incorrectly stated chromium.
A hydrocarbon has the empirical formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\). When 1.17 g of the compound is heated to 85 °C at a pressure of 101 kPa it occupies a volume of \({\text{400 c}}{{\text{m}}^{\text{3}}}\).
(i) Calculate the molar mass of the compound, showing your working.
(ii) Deduce the molecular formula of the compound.
\({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) exists as three isomers. Identify the structure of the isomer with the lowest boiling point and explain your choice.
Ethanol is a primary alcohol that can be oxidized by acidified potassium dichromate(VI). Distinguish between the reaction conditions needed to produce ethanal and ethanoic acid.
Ethanal:
Ethanoic acid:
Determine the oxidation number of carbon in ethanol and ethanal.
Ethanol:
Ethanal:
Deduce the half-equation for the oxidation of ethanol to ethanal.
Deduce the overall redox equation for the reaction of ethanol to ethanal with acidified potassium dichromate(VI) by combining your answer to part (c) (iii) with the following half-equation:
\[{\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }{\text{(aq)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ - } \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
Describe two characteristics of a reaction at equilibrium.
Describe how a catalyst increases the rate of a reaction.
State and explain the effect of a catalyst on the position of equilibrium.
Ethanoic acid reacts with ethanol to form the ester ethyl ethanoate.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH(l)}} + {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH(l)?????C}}{{\text{H}}_{\text{3}}}{\text{COOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{(l)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
The esterification reaction is exothermic. State the effect of increasing temperature on the value of the equilibrium constant (\({K_{\text{c}}}\)) for this reaction.
Markscheme
(i) \({\text{temperature}} = 358{\text{ K}}\);
\(M = \frac{{mRT}}{{pV}}/1.17 \times 8.31 \times \frac{{358}}{{(0.40 \times 101)}}\);
\({\text{(}}M = {\text{) }}86.2{\text{ (gmo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [1 max] for correct final answer without working.
(ii) \({{\text{C}}_6}{{\text{H}}_{14}}\);
\({\text{C(C}}{{\text{H}}_3}{{\text{)}}_4}\);
Accept correct name 2,2-dimethylpropane.
Do not penalize missing H atoms.
weakest London/dispersion/van der Waals’/vdW/instantaneous induced dipoleinduced dipole forces because of smallest surface area/contact
OR
weakest London/dispersion/van der Waals’/vdW/ instantaneous induced dipoleinduced dipole forces because of least distortion of the electron cloud
OR
weakest London/dispersion/van der Waals’/vdW/ instantaneous induced dipoleinduced dipole forces because polarizability of electrons (in electron cloud) is less;
Accept other words to that effect but student must mention a correct IMF and a correct reason.
Ethanal: distill off product as it forms;
Accept distillation.
Ethanoic acid: (heat under) reflux / use excess oxidizing agent;
Ethanol: –2/–II;
Ethanal: –1/–I;
Do not accept 2– or 1–, but penalize only once.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} \to {\text{C}}{{\text{H}}_3}{\text{CHO}} + {\text{2}}{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - }\);
Half-equation required. Do not accept \({C_2}{H_5}OH + 2[O] \to C{H_3}CHO + {H_2}O\).
Accept e for \({e^ - }\).
\({\text{3C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH(aq)}} + {\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{{\text{3}} + }}{\text{(aq)}} + {\text{3C}}{{\text{H}}_3}{\text{CHO(l)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\)
correct reactants and products;
correct balancing;
M2 can only be scored if M1 correct.
Ignore state symbols.
rate of forward process/reaction = rate of backward/reverse process/reaction;
concentrations of reactants and products remain constant;
no change in macroscopic properties;
closed/isolated system / constant matter/energy;
provides alternative pathway (of lower energy);
lowers activation energy (of the reaction) / more particles with \(E \geqslant {E_{\text{a}}}\);
no effect (on position of equilibrium);
increases rate of forward and reverse reactions (equally);
decreases;
Examiners report
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
This was the least popular question however many who chose it were successful in parts. Part (a) that required a calculation of \({M_{\text{r}}}\) was quite well done. However (b) that asked for the isomer of \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) with the lowest boiling point was not well answered. Identification of the methods to produce ethanal or ethanoic acid was done well by the strong candidates and others just guessed. Deduction of oxidation numbers and then writing of redox equations was not well answered. However (d) and (e) about equilibrium were answered well by many candidates, although there were again some very poor answers.
In acidic solution, ions containing titanium can react according to the half-equation below.
\[{\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\]
A reactivity series comparing titanium, cadmium and europium is given below.
Least reactive Cd \( < \) Ti \( < \) Eu Most reactive
The half-equations corresponding to these metals are:
\({\text{E}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Eu(s)}}\)
\({\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {\text{3}}{{\text{e}}^ - } \rightleftharpoons {\text{Ti(s)}}\)
\({\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Cd(s)}}\)
Some students were provided with a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a monobasic acid, HQ, and given the problem of determining whether HQ was a weak acid or a strong acid.
State the initial and final oxidation numbers of titanium and hence deduce whether it is oxidized or reduced in this change.

Considering the above equilibrium, predict, giving a reason, how adding more acid would affect the strength of the \({\text{Ti}}{{\text{O}}^{2 + }}\) ion as an oxidizing agent.
Deduce which of the species would react with titanium metal.
Deduce the balanced equation for this reaction.
Deduce which of the six species is the strongest oxidizing agent.
A voltaic cell can be constructed using cadmium and europium half-cells. State how the two solutions involved should be connected and outline how this connection works.
Define a Brønsted–Lowry acid.
Distinguish between the terms strong acid and weak acid.
Neelu and Charles decided to solve the problem by determining the volume of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution needed to neutralize \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of the acid. Outline whether this was a good choice.
Neelu and Charles decided to compare the volume of sodium hydroxide solution needed with those required by known \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) strong and weak acids. Unfortunately they chose sulfuric acid as the strong acid. Outline why this was an unsuitable choice.
State a suitable choice for both the strong acid and the weak acid.
Strong acid:
Weak acid:
Francisco and Shamiso decided to measure the pH of the initial solution, HQ, and they found that its pH was 3.7. Deduce, giving a reason, the strength (weak or strong) of the acid HQ.
Suggest a method, other than those mentioned above, that could be used to solve the problem and outline how the results would distinguish between a strong acid and a weak acid.
Markscheme

+ sign must be present. Do not award mark for incorrect notation 4, 4+, 3, 3+ etc.
Do not award M2 if inconsistent with M1.
increases / makes it stronger;
(more \({{\text{H}}^ + }\) would) drive/shift equilibrium to the right/towards products
(accepting more electrons);
\({\text{C}}{{\text{d}}^{2 + }}\);
Do not allow incorrect notation such as Cd, Cd(II), or Cd+2.
\({\text{2Ti(s)}} + {\text{3C}}{{\text{d}}^{2 + }}{\text{(aq)}} \to {\text{2T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {\text{3Cd(s)}}\);
Ignore state symbols.
Allow ECF from (b)(i) for a correct equation.
\({\text{C}}{{\text{d}}^{2 + }}\);
Charge must be given.
Do not allow incorrect notation such as Cd, Cd(II), or Cd+2 but penalize
only once in b(i) and b(iii) .
Allow ECF, if Eu2+ is written both in part (i) and part (iii).
salt bridge;
Accept specific examples of salt bridges, such as filter paper dipped in aqueous KNO3.
allows the movement of ions (between the two solutions) / completes the circuit / maintains electrical neutrality;
Accept movement of charges/negative ions/positive ions.
donates \({{\text{H}}^ + }\)/protons;
strong acid completely/100%/fully dissociated/ionized and weak acid partially/slightly dissociated/ionized;
not a good choice / poor choice;
requires same volume of the base / the amount/volume to react/for neutralization does not depend on the acid strength;
sulfuric acid is diprotic/dibasic/liberates two protons/\({{\text{H}}^ + }\);
Accept “reacts with 2 moles of alkali/base”.
Strong acid: hydrochloric acid/HCl / nitric acid/\({\text{HN}}{{\text{O}}_{\text{3}}}\);
Weak acid: ethanoic acid/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\);
Allow acetic acid for weak acid.
Accept any other strong/weak monobasic acids as appropriate.
Do not accept non-monobasic acids, such as phosphoric acid and carbonic acid.
weak;
strong \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) acid has a pH of 1/lower than that observed;
Accept “pH value of 3.7 means that it produces only 10–3.7/2.0 \( \times \) 10–4 [H+] in water”.
measure the rate of reaction with reactive metal/(metal) carbonate/metal oxide;
strong acid would react faster/more vigorously / weak acid would react slower/less vigorously;
Accept specific substances, such as Mg and CaCO3, which react with acids.
OR
measure conductivity;
higher for strong acid / lower for weak acid;
OR
measure heat/enthalpy of neutralization;
greater for strong acid / lower for weak acid;
Do not accept pH/universal indicator paper.
Examiners report
In part (a) (i), most candidates scored full marks although some candidates continue to write incorrect notation (4, 4+) for oxidation states.
In part (ii), some candidates missed the word equilibrium in the question and hence could not state that equilibrium will shift towards right and strength of oxidizing agent will increase.
In part (b) (i), (iii), the correct answer was \({\text{C}}{{\text{d}}^{2 + }}\) but many candidates wrote Cd, Eu or Ti.
In part (ii), the better candidates wrote the correct balanced chemical equation. Some included electrons in the equation which was surprising and some did not read the question where the reaction with Ti metal was asked.
In part (b) (i), (iii), the correct answer was \({\text{C}}{{\text{d}}^{2 + }}\) but many candidates wrote Cd, Eu or Ti.
In part (iv), many candidates identified the salt bridge but some missed the reference to the movement of ions.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
The periodic table shows the relationship between electron arrangement and the properties of elements and is a valuable tool for making predictions in chemistry.
The word redox comes from a combination of the terms reduction and oxidation. Redox reactions affect our daily lives.
The overall reaction that takes place in a voltaic cell is shown below.
\[{\text{Pb(s)}} + {\text{Pb}}{{\text{O}}_{\text{2}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}} \to {\text{2PbS}}{{\text{O}}_{\text{4}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Identify the property used to arrange the elements in the periodic table.
(ii) Outline two reasons why electronegativity increases across period 3 in the periodic table and one reason why noble gases are not assigned electronegativity values.
(i) Define the term first ionization energy of an atom.
(ii) Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
(iii) Explain why sodium conducts electricity but phosphorus does not.
(i) Determine the oxidation number of lead in Pb, \({\text{Pb}}{{\text{O}}_{\text{2}}}\) and \({\text{PbS}}{{\text{O}}_{\text{4}}}\).
(ii) Deduce the oxidation and reduction half-equations taking place at the negative lead electrode (anode) and the positive lead(IV) oxide electrode (cathode). Deduce the oxidizing and reducing agents and state the direction of the electron flow between the electrodes.
(iii) In order to determine the position of three metals in a reactivity series, the metals were placed in different solutions of metal ions. The table below summarizes whether or not a reaction occurred.

State the equations for the three reactions that take place. Use this information to place the metals Ag, Cu and Pb in a reactivity series, with the strongest reducing agent first, and explain your reasoning.
Markscheme
(i) atomic number / Z;
Accept nuclear charge / number of protons.
(ii) Across period 3:
increasing number of protons / atomic number / Z / nuclear charge;
(atomic) radius/size decreases / same shell/energy level / similar shielding/screening (from inner electrons);
No mark for shielding/screening or shielding/screening increases.
Noble gases:
do not form bonds (easily) / have a full/stable octet/shell/energy level / cannot attract more electrons;
Do not accept “inert” or “unreactive” without reference to limited ability/inability to form bonds or attract electrons.
Accept the following as alternative to M3.
no attraction for electrons/full outer shell / stable/inert/do not form bonds (readily/easily);
(i) energy/enthalpy change/required/needed to remove/knock out an electron (to form +1/uni-positive/\({{\text{M}}^{ + 1}}\) ion);
in the gaseous state;
Award [1] for M(g) \( \to \) M+(g) + e–.
Award [2] for M(g) \( \to \) M+(g) + e– with reference to energy/enthalpy change.
(ii) increasing number of protons/atomic number/Z/nuclear charge;
radius/size decreases / same shell/energy level / similar shielding/screening (from inner electrons);
No mark for shielding/screening or shielding/screening increases.
(iii) Na: delocalized electrons / mobile sea of electrons / sea of electrons free to move;
No mark for just “mobile electrons”.
(i) \({\text{Pb: 0, Pb}}{{\text{O}}_{\text{2}}}{\text{: }} + 4{\text{, PbS}}{{\text{O}}_{\text{4}}}{\text{: }} + 2\);
Need sign for mark.
Do not accept notations such as 4+, 2+ or IV, II.
(ii) Negative/–/anode
\({\text{Pb(s)}} + {\text{SO}}_4^{2 - }{\text{(aq)}} \to {\text{PbS}}{{\text{O}}_4}{\text{(s)}} + {\text{2}}{{\text{e}}^ - }/{\text{Pb(s)}} \to {\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
Positive/+/cathode
\({\text{Pb}}{{\text{O}}_2}{\text{(s)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{SO}}_4^{2 - }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{PbS}}{{\text{O}}_4}{\text{(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}/\)
\({\text{Pb}}{{\text{O}}_2}{\text{(s)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}/\)
\({\text{Pb}}{{\text{O}}_2}{\text{(s)}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{PbS}}{{\text{O}}_4}{\text{(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\);
Accept Pb4+ + 2e– \( \to \) Pb2+.
Ignore state symbols.
Allow e instead of e–.
oxidizing agent is \({\text{Pb}}{{\text{O}}_{\text{2}}}\)/lead(IV) oxide/lead dioxide and reducing agent is Pb/lead;
from negative/–/anode/Pb to positive/+/cathode/\({\text{Pb}}{{\text{O}}_{\text{2}}}\) (through the external circuit/wire);
(iii) \({\text{Pb(s)}} + {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{Cu(s)}}\)
\({\text{Pb(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \to {\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}\)
\({\text{Cu(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}\)
Award [2] for three correct, award [1] for any two correct, one correct scores no mark.
Ignore state symbols.
Penalize unbalanced equations once only.
Pb is a stronger reducing agent than Cu and/or Ag / Pb most reactive as it can reduce/displace both \({\text{C}}{{\text{u}}^{2 + }}\) and \({\text{A}}{{\text{g}}^ + }\);
Cu is a stronger reducing agent than Ag but not Pb / Cu in the middle (of the three) as it can reduce/displace \({\text{A}}{{\text{g}}^ + }\) but not \({\text{P}}{{\text{b}}^{2 + }}\);
Accept converse argument.
Decreasing order: Pb, Cu, Ag / \({\text{Pb}} > {\text{Cu}} > {\text{Ag}}\);
Do not accept: Pb2+, Cu2+, Ag+.
Examiners report
Parts (a) and (b) were reasonably well answered. Generally the understanding of electronegativity was good, but some made the error of stating that it was the attraction of one electron only; others did not clarify that it is the ability of an atom to attract a shared electron pair in a covalent bond. The reason for the increase in electronegativity across period 3 was sometimes incomplete with candidates not mentioning both the increase in number of protons and the decrease of atomic size.
Although the definition of first ionisation energy was generally well known quite a few candidates only gave one reason for the increase across a period and some referred to the number of electrons in the outer shell as a reason for the general increase. Many candidates did not refer to delocalized electrons when explaining the difference in electrical conductivity between sodium and phosphorus.
The oxidation numbers of lead in Pb, \({\text{Pb}}{{\text{O}}_{\text{2}}}\) and \({\text{PbS}}{{\text{O}}_{\text{4}}}\) were given correctly by many candidates; however, incorrect responses included 0, II and IV or as 4+ and 2+.
A common mistake was to give +10 for the oxidation state for lead in lead(IV) sulfate presumably as candidates mistakenly assigned an oxidation number of –2 to sulfur. The half reactions for the lead-acid storage battery proved to be difficult with few being able to deduce the half-equation at the cathode, although \({\text{P}}{{\text{b}}^{4 + }} + {\text{2}}{{\text{e}}^ - } \to {\text{P}}{{\text{b}}^{2 + }}\) was accepted. The number of candidates providing the more accurate version of the reaction at the cathode, namely: \({\text{Pb}}{{\text{O}}_{\text{2}}} + {\text{4}}{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - } \to {\text{P}}{{\text{b}}^{2 + }} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O}}\) was very small. In addition, many candidates interchanged the oxidizing and reducing agents. Most candidates were able to place Ag, Cu and Pb in the correct order of reactivity although equations were often unbalanced or included incorrect ionic charges with species such as \({\text{A}}{{\text{g}}^{2 + }}\) or \({\text{C}}{{\text{u}}^ + }\). Many could not explain the position of metals in the reactivity series clearly with statements such as “Pb is the strongest reducing agent because it reduces Cu and Ag” instead of \({\text{C}}{{\text{u}}^{2 + }}\) and \({\text{A}}{{\text{g}}^ + }\).
Ethanedioic acid (oxalic acid), \({{\text{(COOH)}}_{\text{2}}}\), reacts with acidified potassium permanganate solution, \({\text{KMn}}{{\text{O}}_{\text{4}}}\), according to the following equation.
\[{\text{5(COOH}}{{\text{)}}_2}{\text{(aq)}} + {\text{2MnO}}_4^ - {\text{(aq)}} + {\text{6}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{10C}}{{\text{O}}_2}{\text{(g)}} + {\text{2M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{8}}{{\text{H}}_2}{\text{O(l)}}\]
The reaction is a redox reaction.
Define oxidation in terms of electron transfer.
Calculate the change in oxidation numbers of carbon and manganese.
Carbon:
Manganese:
Identify the oxidizing and reducing agents.
Oxidizing agent:
Reducing agent:
Markscheme
loss of electrons;
Carbon:
III to IV / +3 to +4 / (+)1;
Manganese:
VII to II / +7 to +2 / –5;
Penalize incorrect notation such as 3+ once only.
Oxidizing agent: \({\text{MnO}}_{\text{4}}^ - \) and Reducing agent: \({{\text{(COOH)}}_{\text{2}}}\);
Accept correct names instead of formulas.
Do not accept Mn and C.
Examiners report
Oxidation was generally correctly defined.
Many candidates calculated correctly the change in oxidation numbers, though often 2+ instead of +2 (or II) was given for the oxidation state of \({\text{M}}{{\text{n}}^{2 + }}\).
Marks were lost because candidates did not name the species but the elements or gave incorrect formula of \({{\text{(COOH)}}_{\text{2}}}\) or forgot the sign on \({\text{MnO}}_{\text{4}}^ - \).
Iron tablets are often prescribed to patients. The iron in the tablets is commonly present as iron(II) sulfate, \({\text{FeS}}{{\text{O}}_{\text{4}}}\).
Two students carried out an experiment to determine the percentage by mass of iron in a brand of tablets marketed in Cyprus.
Experimental Procedure:
• The students took five iron tablets and found that the total mass was 1.65 g.
• The five tablets were ground and dissolved in \({\text{100 c}}{{\text{m}}^{\text{3}}}\) dilute sulfuric acid, \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). The solution and washings were transferred to a \({\text{250 c}}{{\text{m}}^{\text{3}}}\) volumetric flask and made up to the mark with deionized (distilled) water.
• \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of this \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) solution was transferred using a pipette into a conical flask. Some dilute sulfuric acid was added.
• A titration was then carried out using a \(5.00 \times {10^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) standard solution of potassium permanganate, \({\text{KMn}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). The end-point of the titration was indicated by a slight pink colour.
The following results were recorded.

This experiment involves the following redox reaction.
\[{\text{5F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{MnO}}_4^ - {\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{5F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
When the \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) solution was made up in the \({\text{250 c}}{{\text{m}}^{\text{3}}}\) volumetric flask, deionized (distilled) water was added until the bottom of its meniscus corresponded to the graduation mark on the flask. It was noticed that one of the two students measured the volume of the solution from the top of the meniscus instead of from the bottom. State the name of this type of error.
State what is meant by the term precision.
When the students recorded the burette readings, following the titration with KMnO4 (aq),the top of the meniscus was used and not the bottom. Suggest why the students read the top of the meniscus and not the bottom.
Define the term reduction in terms of electrons.
Deduce the oxidation number of manganese in the \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\) ion.
Determine the amount, in mol, of \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\), used in each accurate titre.
Calculate the amount, in mol, of \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) ions in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the solution.
Determine the total mass of iron, in g, in the \({\text{250 c}}{{\text{m}}^{\text{3}}}\) solution.
Determine the percentage by mass of iron in the tablets.
One titration was abandoned because a brown precipitate, manganese(IV) oxide, formed. State the chemical formula of this compound.
Markscheme
systematic (error);
Do not accept parallax.
closeness of agreement of a set of measurements to each other / OWTTE;
Allow reproducibility/consistency of measurement / measurements with small random errors/total amount of random errors/standard deviation / a more precise value contains more significant figures / OWTTE.
potassium permanganate has a very dark/deep (purple) colour so cannot read bottom of meniscus / OWTTE;
gain (of electrons);
VII / +7;
Do not accept 7 or 7+.
volume \( = 16.80{\text{ }}({\text{c}}{{\text{m}}^3})/18.00 - 1.20{\text{ }}({\text{c}}{{\text{m}}^3})\);
amount \(\left( { = \frac{{16.80 \times 5.00 \times {{10}^{ - 3}}}}{{1000}}} \right) = 8.40 \times {10^{ - 5}}{\text{ (mol)}}\);
Award [2] for correct final answer.
\((8.40 \times {10^{ - 5}} \times 5 \times 10) = 4.20 \times {10^{ - 3}}{\text{ (mol per 250 c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
\((55.85 \times 4.20 \times {10^{ - 3}}) = 0.235{\text{ (g)}}\);
Do not penalize if 56 g mol–1 is used for atomic mass of iron.
\(\left( {\frac{{{\text{0.235}} \times {\text{100}}}}{{{\text{1.65}}}} = } \right){\text{ 14.2% }}\);
No ECF if answer >100 %.
MnO2;
Examiners report
Question 1 presented difficulties to many candidates. It is felt that the extended nature of the response distracted candidates from rather straight-forward quantitative chemistry calculations. Part (a) required candidates to determine whether an error was systematic or random and part (b) asked for the meaning of precision. Both of these questions are relevant to Topic 11.
Question 1 presented difficulties to many candidates. It is felt that the extended nature of the response distracted candidates from rather straight-forward quantitative chemistry calculations. Part (a) required candidates to determine whether an error was systematic or random and part (b) asked for the meaning of precision. Both of these questions are relevant to Topic 11.
Very few candidates related reading the top of the meniscus in the burette in part (c) to the colour of the KMnO4 solution. While it is acknowledged that few candidates would have performed this experiment themselves, it is reasonable that candidates should know the colour of KMnO4.
Part d) (i) was answered very well with nearly all candidates correctly defining reduction.
In d) (ii) many candidates correctly deduced the oxidation number of Mn in MnO4. Several lost marks, however, for not using acceptable notation. 7 by itself is not correct.
Part (e) involved the calculations. Candidates were guided through the process of calculating number of moles from concentration and volume, finding mole ratios, and determining mass from moles and molar mass. Better candidates performed these calculations well. Weaker candidates often scored follow-through marks when working was shown.
Part (e) involved the calculations. Candidates were guided through the process of calculating number of moles from concentration and volume, finding mole ratios, and determining mass from moles and molar mass. Better candidates performed these calculations well. Weaker candidates often scored follow-through marks when working was shown.
Part (e) involved the calculations. Candidates were guided through the process of calculating number of moles from concentration and volume, finding mole ratios, and determining mass from moles and molar mass. Better candidates performed these calculations well. Weaker candidates often scored follow-through marks when working was shown.
Part (e) involved the calculations. Candidates were guided through the process of calculating number of moles from concentration and volume, finding mole ratios, and determining mass from moles and molar mass. Better candidates performed these calculations well. Weaker candidates often scored follow-through marks when working was shown.
In f) (i) a common error was to write Mn2O4 as the formula for manganese(IV) oxide. Also common was the use of the symbol Mg for manganese.
Bromomethane was used as a pesticide until it was found to be ozone-depleting.
State the equation for the reaction between methane and bromine to form bromomethane.
Explain, using equations, the complete free-radical mechanism for the reaction of methane with bromine, including necessary reaction conditions.
Bromine can be produced by the electrolysis of molten sodium bromide. Deduce the half-equation for the reaction at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
Bromine reacts with aqueous sodium iodide:
\[{\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}} + {\text{2NaI(aq)}} \to {{\text{I}}_{\text{2}}}{\text{(aq)}} + {\text{2NaBr(aq)}}\]
Identify the oxidizing agent in this reaction.
Markscheme
\({\text{C}}{{\text{H}}_{\text{4}}} + {\text{B}}{{\text{r}}_{\text{2}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}} + {\text{HBr }}\);
Initiation:
\({\text{B}}{{\text{r}}_2}\xrightarrow{{UV/hf/hv}}2{\text{BR}} \bullet \);
Reference to UV/light or high temperatures must be included.
Propagation:
\({\text{Br}} \bullet + {\text{C}}{{\text{H}}_4} \to {\text{C}}{{\text{H}}_3} \bullet + {\text{HBr}}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{B}}{{\text{r}}_2} \to {\text{C}}{{\text{H}}_3}{\text{Br}} + {\text{Br}} \bullet \);
Termination:
Award [1 max] for any one of:
\({\text{Br}} \bullet + {\text{Br}} \bullet \to {\text{B}}{{\text{r}}_2}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{Br}} \bullet \to {\text{C}}{{\text{H}}_3}{\text{Br}}\);
\({\text{C}}{{\text{H}}_3} \bullet + {\text{C}}{{\text{H}}_3} \bullet \to {{\text{C}}_2}{{\text{H}}_6}\);
Accept representation of radical without \( \bullet \) (eg Br, CH3) if consistent throughout mechanism.
Award [3 max] if initiation, propagation and termination are not stated or are incorrectly labelled for equations.
Positive electrode (anode):
\({\text{2B}}{{\text{r}}^ - } \to {\text{B}}{{\text{r}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }/{\text{B}}{{\text{r}}^ - } \to \frac{1}{2}{\text{B}}{{\text{r}}_2}{\text{(g)}} + {{\text{e}}^ - }\);
Negative electrode (cathode):
\({\text{N}}{{\text{a}}^ + } + {{\text{e}}^ - } \to {\text{Na(l)}}\);
Award [1 max] for correct equations at incorrect electrodes.
Ignore state symbols.
Accept e instead of \({e^ - }\).
Penalize use of equilibrium signs once only.
bromine/\({\text{B}}{{\text{r}}_{\text{2}}}\);
Do not accept bromide/\(B{r^ - }\).
Examiners report
Part (a) and (b) of the question were based on the free radical substitution of methane and typical marks were 0/5 or 5/5, as it required the stating of a mechanism that has been on the syllabus and on examination papers for many years. Students generally had little idea but some had obviously learned it well. The students who gained some marks often lost marks for creating hydrogen radicals. The rest of the question was based on redox and (c) which asked for the equations at the electrodes during the electrolysis of sodium bromide was done very badly indeed. These half-equations are in the data booklet and yet very few students could come up with the correct equations. Some students had NaBr at both electrodes. However many students did correctly identify bromine as the oxidizing agent in (d).
Part (a) and (b) of the question were based on the free radical substitution of methane and typical marks were 0/5 or 5/5, as it required the stating of a mechanism that has been on the syllabus and on examination papers for many years. Students generally had little idea but some had obviously learned it well. The students who gained some marks often lost marks for creating hydrogen radicals. The rest of the question was based on redox and (c) which asked for the equations at the electrodes during the electrolysis of sodium bromide was done very badly indeed. These half-equations are in the data booklet and yet very few students could come up with the correct equations. Some students had NaBr at both electrodes. However many students did correctly identify bromine as the oxidizing agent in (d).
Part (a) and (b) of the question were based on the free radical substitution of methane and typical marks were 0/5 or 5/5, as it required the stating of a mechanism that has been on the syllabus and on examination papers for many years. Students generally had little idea but some had obviously learned it well. The students who gained some marks often lost marks for creating hydrogen radicals. The rest of the question was based on redox and (c) which asked for the equations at the electrodes during the electrolysis of sodium bromide was done very badly indeed. These half-equations are in the data booklet and yet very few students could come up with the correct equations. Some students had NaBr at both electrodes. However many students did correctly identify bromine as the oxidizing agent in (d).
Part (a) and (b) of the question were based on the free radical substitution of methane and typical marks were 0/5 or 5/5, as it required the stating of a mechanism that has been on the syllabus and on examination papers for many years. Students generally had little idea but some had obviously learned it well. The students who gained some marks often lost marks for creating hydrogen radicals. The rest of the question was based on redox and (c) which asked for the equations at the electrodes during the electrolysis of sodium bromide was done very badly indeed. These half-equations are in the data booklet and yet very few students could come up with the correct equations. Some students had NaBr at both electrodes. However many students did correctly identify bromine as the oxidizing agent in (d).
Electrolysis is an important industrial process used to obtain very reactive elements from their common ores.
Molten magnesium chloride can be electrolysed using inert graphite electrodes at 800 °C.
Describe, using a labelled diagram, the essential components of this electrolytic cell.
Molten magnesium chloride can be electrolysed using inert graphite electrodes at 800 °C.
Deduce the half-equations, including state symbols, for the reactions occurring at each electrode. (The melting points of MgCl2 and Mg are 714 °C and 649 °C respectively.)
Positive electrode (anode):
Negative electrode (cathode):
Outline why solid magnesium chloride does not conduct electricity.
Aluminium can also be obtained by electrolysis. Suggest one reason why aluminium is often used instead of iron by engineers.
Markscheme
Cell showing:
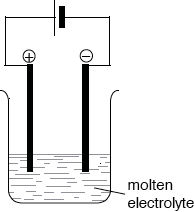
molten electrolyte/MgCl2(l), electrodes and battery/DC supply;
correct labelling of positive electrode/anode/+ and negative electrode/cathode/–;
Positive electrode (anode):
\(2{\text{C}}{{\text{l}}^ - }{\text{(l)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + 2{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - }{\text{(l)}} \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}} + {{\text{e}}^ - }\);
Negative electrode (cathode):
\({\text{M}}{{\text{g}}^{2 + }}{\text{(l)}} + 2{{\text{e}}^ - } \to {\text{Mg(l)}}\);
Accept e instead of e–.
Award [1 max] for correct half-equations given at the wrong electrode.
Penalize use of reversible arrows once only.
correct state symbols in both equations;
ions are not free to move when solid / ions in rigid lattice / OWTTE;
aluminium/Al is less dense (compared to iron/Fe) / Al is more ductile or malleable/ aluminium forms a protective oxide layer / Al does not corrode / iron/Fe rusts /OWTTE;
Do not accept “Al is lighter” OR “less expensive” OR “Al can be recycled”.
Examiners report
There were very few carefully drawn correct diagrams as well as too many diagrams showing half-cells. The importance of the solution being molten was not appreciated. The equations did pick up marks, but it was extremely rare for candidates to access the mark for the correct state symbols. Far too many associated electrical conductivity in molten compounds with mobile electrons. The awareness that mobile ions are responsible for conductivity was poorly understood. The difference between "lightness" and density is still confused.
There were very few carefully drawn correct diagrams as well as too many diagrams showing half-cells. The importance of the solution being molten was not appreciated. The equations did pick up marks, but it was extremely rare for candidates to access the mark for the correct state symbols. Far too many associated electrical conductivity in molten compounds with mobile electrons. The awareness that mobile ions are responsible for conductivity was poorly understood. The difference between "lightness" and density is still confused.
There were very few carefully drawn correct diagrams as well as too many diagrams showing half-cells. The importance of the solution being molten was not appreciated. The equations did pick up marks, but it was extremely rare for candidates to access the mark for the correct state symbols. Far too many associated electrical conductivity in molten compounds with mobile electrons. The awareness that mobile ions are responsible for conductivity was poorly understood. The difference between "lightness" and density is still confused.
There were very few carefully drawn correct diagrams as well as too many diagrams showing half-cells. The importance of the solution being molten was not appreciated. The equations did pick up marks, but it was extremely rare for candidates to access the mark for the correct state symbols. Far too many associated electrical conductivity in molten compounds with mobile electrons. The awareness that mobile ions are responsible for conductivity was poorly understood. The difference between "lightness" and density is still confused.
Both sodium and sodium chloride can conduct electricity.
Compare how electric current passes through sodium and sodium chloride by completing the table below.

Sodium can be obtained by electrolysis from molten sodium chloride. Describe, using a diagram, the essential components of this electrolytic cell.
Markscheme

Award [1] for each feature that is correct for both sodium and sodium chloride.
Accept equation or half-equations for the reaction of sodium chloride in “reaction occurring”.

clear diagram containing all elements (power supply, connecting wires, electrodes, container and electrolyte);
labelled positive electrode/anode and negative electrode/cathode;
Accept positive and negative by correct symbols near power supply.
Accept power supply if shown as conventional long/short lines (as in diagram above) or clearly labelled DC power supply.
labelled electrolyte/NaCl(l);
State of NaCl not needed.
Examiners report
Very poorly answered. The state of matter received most marks, conducting particles seldom correct and reaction occurring generally misunderstood by candidates.
Diagrams were very poorly drawn, many without power supplies and the wires within the electrolyte. The electrodes were often mis-signed as Na and/or Cl. Many candidates seem to confuse voltaic cells with electrolytic cells.
Oxidation and reduction can be defined in terms of electron transfer or oxidation numbers.
Alcohols with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) occur as four structural isomers. Three of the isomers can be oxidized with acidified potassium dichromate solution to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). The half-equation for the dichromate ion is:
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ - } \rightleftharpoons {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\]
Electrolysis has made it possible to obtain reactive metals from their ores.
A reactivity series can be experimentally determined by adding the metals W, X, Y and Z to solutions of these metal ions. The following reactions were observed:
\({{\text{W}}^{2 + }}{\text{(aq)}} + {\text{X(s)}} \to {\text{W(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{W(s)}}\)
\({{\text{Z}}^{2 + }}{\text{(aq)}} + {\text{W(s)}} \to {\text{Z(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{X(s)}}\)
Define oxidation in terms of electron transfer.
(i) Deduce the oxidation number of chromium in \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }\).
(ii) Deduce the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(iii) Deduce the overall equation for the redox reaction.
(iv) Two of the isomers with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) can be oxidized further to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\). Deduce the structural formulas of these two isomers.
(v) One isomer cannot be oxidized by acidified potassium dichromate solution.
Deduce its structural formula, state its name and identify it as a primary, secondary or tertiary alcohol.
Name:
Alcohol:
(vi) All isomers of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) undergo complete combustion. State an equation for the complete combustion of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(i) Draw a labelled electrolytic cell for the electrolysis of molten potassium bromide, KBr. Include the direction of electron flow, the positive electrode (anode) and the negative electrode (cathode), the location of oxidation and reduction, and the electrolyte.
(ii) Deduce a half-equation for the reaction that occurs at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
(iii) Describe how current is conducted in a molten electrolyte.
(i) Deduce the order of reactivity of these four metals, from the least to the most reactive.
(ii) A voltaic cell is made by connecting a half-cell of X in \({\text{XC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\) to a half-cell of Z in \({\text{ZC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\). Deduce the overall equation for the reaction taking place when the cell is operating.
Markscheme
loss of electrons;
(i) \( + 6{\text{/VI}}\);
Do not award mark if incorrect notation used, ie, 6 , 6+ or –6.
(ii) \({{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} \to {{\text{C}}_4}{{\text{H}}_8}{\text{O(l)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
Ignore state symbols.
(iii) \({\text{3}}{{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} + {\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{3}}{{\text{C}}_4}{{\text{H}}_8}{\text{O(l)}} + {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
(iv) \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{OH}}\);
\({{\text{(C}}{{\text{H}}_3}{\text{)}}_2}{\text{CHC}}{{\text{H}}_2}{\text{OH}}\);
Accept full or condensed structural formulas.
(v) \({{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{COH}}\);
2-methylpropan-2-ol;
Allow 2-methyl-2-propanol , methylpropan-2-ol, methyl-2-propanol.
tertiary;
(vi) \({{\text{C}}_4}{{\text{H}}_9}{\text{OH}} + {\text{6}}{{\text{O}}_2} \to {\text{4C}}{{\text{O}}_2} + {\text{5}}{{\text{H}}_2}{\text{O}}/{{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{COH}} + {\text{6}}{{\text{O}}_2} \to {\text{4C}}{{\text{O}}_2} + {\text{5}}{{\text{H}}_2}{\text{O}}\)
correct reactants and products;
correct balancing;
(i) (DC) power supply

(DC) power supply / battery;
electrodes labelled as +/anode or –/cathode and electron flow;
reduction at negative electrode (cathode) / oxidation at positive electrode (anode);
electrolyte / molten KBr/KBr(l) / \({{\text{K}}^ + }{\text{(l)}}\) and \({\text{B}}{{\text{r}}^ - }{\text{(l)}}\);
(ii) Positive electrode (anode):
\({\text{2B}}{{\text{r}}^ - }{\text{(l)}} \to {\text{B}}{{\text{r}}_2}{\text{(l)}} + {\text{2}}{{\text{e}}^ - }\);
Negative electrode (cathode):
\({{\text{K}}^ + }{\text{(l)}} + {{\text{e}}^ - } \to {\text{K(l)}}\);
Award [1 max] if correct half-equations are given at the wrong electrodes.
Allow e instead of e–.
Ignore state symbols.
Penalize equilibrium sign once only.
(iii) positive ions move towards negative electrode (cathode) and negative ions move towards positive electrode (anode) / ions move to oppositely charged electrode / negative ions give up electrons at positive electrode and positive ions gain electrons at negative electrode;
(i) \({\text{Z}} < {\text{W}} < {\text{X}} < {\text{Y}}\);
Accept \(Y > X > W > Z\).
(ii) \({\text{X(s)}} + {{\text{Z}}^{2 + }}{\text{(aq)}} \to {{\text{X}}^{2 + }}{\text{(aq)}} + {\text{Z(s)}}\);
Ignore state symbols.
Accept X(s) + ZCl2(aq) \( \to \) XCl2(aq) + Z(s).
Examiners report
This was another popular choice of question in Section B. In part (a) almost all candidates defined oxidation correctly. The oxidation number of chromium was mostly determined correctly in (b)(i), but only the better candidates could write the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) in (b)(ii), even though the product was identified in the question as \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Subsequently the overall equation of the redox reaction in (b)(iii) was poorly answered. One respondent stated that balanced redox equations are not required. This is stated in 9.2.2. In (b)(iv), candidates who realized the product with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) was an acid, deduced correct formulas of the two primary alcohols, though some did not read the question and gave the formulae for the acid and not the alcohol. Commonly, candidates drew isomers of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) giving one primary structure and one secondary structure. Incorrect structures frequently had oxygen atoms connected to the molecule with single bonds but nothing else attached. Part (v) required candidates to identify the isomer which cannot be oxidized by acidified potassium dichromate solution. Many candidates correctly gave the formula and name of the tertiary alcohol. In (b)(vi) several candidates gave a correct equation for the combustion of alcohols but more usually one mark was scored for correct reactants and products and the mark for correct balancing was missed. Part (c) was on electrolysis. There were several poorly drawn electrolytic cells in (c)(i), sometimes even with the electrodes outside of the electrolyte, but most candidates managed a few marks and many candidates scored full marks. A significant number of candidates drew a voltaic cell with a salt bridge and a small minority had the battery terminals incorrectly connected or drew a voltmeter. The half-equations for electrode reactions were poorly done in (c)(ii) with several candidates again writing whole equations. The reduction of the potassium ion was often given at the anode and the oxidation of the bromide ion was seldom done well with many candidates writing a reduction half-equation for Br2. In (c)(iii) candidates described poorly how current is conducted in a molten electrolyte. The common response was that electrons are forced through the solution from the cathode to the anode. Many candidates deduced a correct order of reactivity for the metals listed in (d)(i) but the overall equation for the reaction occurring in a voltaic cell made from two of the metals was either done well or was completely wrong in (d)(ii).
This was another popular choice of question in Section B. In part (a) almost all candidates defined oxidation correctly. The oxidation number of chromium was mostly determined correctly in (b)(i), but only the better candidates could write the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) in (b)(ii), even though the product was identified in the question as \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Subsequently the overall equation of the redox reaction in (b)(iii) was poorly answered. One respondent stated that balanced redox equations are not required. This is stated in 9.2.2. In (b)(iv), candidates who realized the product with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) was an acid, deduced correct formulas of the two primary alcohols, though some did not read the question and gave the formulae for the acid and not the alcohol. Commonly, candidates drew isomers of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) giving one primary structure and one secondary structure. Incorrect structures frequently had oxygen atoms connected to the molecule with single bonds but nothing else attached. Part (v) required candidates to identify the isomer which cannot be oxidized by acidified potassium dichromate solution. Many candidates correctly gave the formula and name of the tertiary alcohol. In (b)(vi) several candidates gave a correct equation for the combustion of alcohols but more usually one mark was scored for correct reactants and products and the mark for correct balancing was missed. Part (c) was on electrolysis. There were several poorly drawn electrolytic cells in (c)(i), sometimes even with the electrodes outside of the electrolyte, but most candidates managed a few marks and many candidates scored full marks. A significant number of candidates drew a voltaic cell with a salt bridge and a small minority had the battery terminals incorrectly connected or drew a voltmeter. The half-equations for electrode reactions were poorly done in (c)(ii) with several candidates again writing whole equations. The reduction of the potassium ion was often given at the anode and the oxidation of the bromide ion was seldom done well with many candidates writing a reduction half-equation for Br2. In (c)(iii) candidates described poorly how current is conducted in a molten electrolyte. The common response was that electrons are forced through the solution from the cathode to the anode. Many candidates deduced a correct order of reactivity for the metals listed in (d)(i) but the overall equation for the reaction occurring in a voltaic cell made from two of the metals was either done well or was completely wrong in (d)(ii).
This was another popular choice of question in Section B. In part (a) almost all candidates defined oxidation correctly. The oxidation number of chromium was mostly determined correctly in (b)(i), but only the better candidates could write the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) in (b)(ii), even though the product was identified in the question as \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Subsequently the overall equation of the redox reaction in (b)(iii) was poorly answered. One respondent stated that balanced redox equations are not required. This is stated in 9.2.2. In (b)(iv), candidates who realized the product with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) was an acid, deduced correct formulas of the two primary alcohols, though some did not read the question and gave the formulae for the acid and not the alcohol. Commonly, candidates drew isomers of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) giving one primary structure and one secondary structure. Incorrect structures frequently had oxygen atoms connected to the molecule with single bonds but nothing else attached. Part (v) required candidates to identify the isomer which cannot be oxidized by acidified potassium dichromate solution. Many candidates correctly gave the formula and name of the tertiary alcohol. In (b)(vi) several candidates gave a correct equation for the combustion of alcohols but more usually one mark was scored for correct reactants and products and the mark for correct balancing was missed. Part (c) was on electrolysis. There were several poorly drawn electrolytic cells in (c)(i), sometimes even with the electrodes outside of the electrolyte, but most candidates managed a few marks and many candidates scored full marks. A significant number of candidates drew a voltaic cell with a salt bridge and a small minority had the battery terminals incorrectly connected or drew a voltmeter. The half-equations for electrode reactions were poorly done in (c)(ii) with several candidates again writing whole equations. The reduction of the potassium ion was often given at the anode and the oxidation of the bromide ion was seldom done well with many candidates writing a reduction half-equation for Br2. In (c)(iii) candidates described poorly how current is conducted in a molten electrolyte. The common response was that electrons are forced through the solution from the cathode to the anode. Many candidates deduced a correct order of reactivity for the metals listed in (d)(i) but the overall equation for the reaction occurring in a voltaic cell made from two of the metals was either done well or was completely wrong in (d)(ii).
This was another popular choice of question in Section B. In part (a) almost all candidates defined oxidation correctly. The oxidation number of chromium was mostly determined correctly in (b)(i), but only the better candidates could write the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) in (b)(ii), even though the product was identified in the question as \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Subsequently the overall equation of the redox reaction in (b)(iii) was poorly answered. One respondent stated that balanced redox equations are not required. This is stated in 9.2.2. In (b)(iv), candidates who realized the product with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\) was an acid, deduced correct formulas of the two primary alcohols, though some did not read the question and gave the formulae for the acid and not the alcohol. Commonly, candidates drew isomers of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) giving one primary structure and one secondary structure. Incorrect structures frequently had oxygen atoms connected to the molecule with single bonds but nothing else attached. Part (v) required candidates to identify the isomer which cannot be oxidized by acidified potassium dichromate solution. Many candidates correctly gave the formula and name of the tertiary alcohol. In (b)(vi) several candidates gave a correct equation for the combustion of alcohols but more usually one mark was scored for correct reactants and products and the mark for correct balancing was missed. Part (c) was on electrolysis. There were several poorly drawn electrolytic cells in (c)(i), sometimes even with the electrodes outside of the electrolyte, but most candidates managed a few marks and many candidates scored full marks. A significant number of candidates drew a voltaic cell with a salt bridge and a small minority had the battery terminals incorrectly connected or drew a voltmeter. The half-equations for electrode reactions were poorly done in (c)(ii) with several candidates again writing whole equations. The reduction of the potassium ion was often given at the anode and the oxidation of the bromide ion was seldom done well with many candidates writing a reduction half-equation for Br2. In (c)(iii) candidates described poorly how current is conducted in a molten electrolyte. The common response was that electrons are forced through the solution from the cathode to the anode. Many candidates deduced a correct order of reactivity for the metals listed in (d)(i) but the overall equation for the reaction occurring in a voltaic cell made from two of the metals was either done well or was completely wrong in (d)(ii).
The Haber process enables the large-scale production of ammonia needed to make fertilizers.
The equation for the Haber process is given below.
\[{{\text{N}}_2}({\text{g)}} + 3{{\text{H}}_2}({\text{g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}\]
The percentage of ammonia in the equilibrium mixture varies with temperature.

Fertilizers may cause health problems for babies because nitrates can change into nitrites in water used for drinking.
A student decided to investigate the reactions of the two acids with separate samples of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.
(i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and explain your choice.
(ii) State and explain the effect of increasing the pressure on the yield of ammonia.
(iii) Explain the effect of increasing the temperature on the rate of reaction.
(i) Define oxidation in terms of oxidation numbers.
(ii) Deduce the oxidation states of nitrogen in the nitrate, \({\text{NO}}_{\text{3}}^ - \), and nitrite, \({\text{NO}}_{\text{2}}^ - \), ions.
The nitrite ion is present in nitrous acid, HNO2, which is a weak acid. The nitrate ion is present in nitric acid, HNO3, which is a strong acid. Distinguish between the terms strong and weak acid and state the equations used to show the dissociation of each acid in aqueous solution.
A small piece of magnesium ribbon is added to solutions of nitric and nitrous acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids.
(i) Calculate the volume of the sodium hydroxide solution required to react exactly with a \({\text{15.0 c}}{{\text{m}}^{\text{3}}}\) solution of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid.
(ii) The following hypothesis was suggested by the student: “Since nitrous acid is a weak acid it will react with a smaller volume of the \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.” Comment on whether or not this is a valid hypothesis.
The graph below shows how the conductivity of the two acids changes with concentration.

Identify Acid 1 and explain your choice.
Nitric acid reacts with silver in a redox reaction.
__ \({\text{Ag(s)}} + \) __ \({\text{NO}}_3^ - {\text{(aq)}} + \) ___ \( \to \) ___\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + \) __ \({\text{NO(g)}} + \) ____
Using oxidation numbers, deduce the complete balanced equation for the reaction showing all the reactants and products.
Markscheme
(i) exothermic;
Accept either of the following for the second mark.
increasing temperature favours endothermic/reverse reaction;
as yield decreases with increasing temperature;
(ii) yield increases / equilibrium moves to the right / more ammonia;
increase in pressure favours the reaction which has fewer moles of gaseous products;
(iii) (rate increases because) increase in frequency (of collisions);
increase in energy (of collisions);
more colliding molecules with \(E \geqslant {E_{\text{a}}}\);
(i) increase in the oxidation number;
(ii) (NO3) + 5 and (NO2–) + 3;
Accept V and III.
Do not penalize missing charges on numbers.
strong acid completely dissociated/ionized and weak acid partially dissociated/ionized;
\({\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {{\text{H}}^ + }{\text{(aq)}} + {\text{NO}}_3^ - {\text{(aq)}}\);
\({\text{HN}}{{\text{O}}_2}{\text{(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{NO}}_2^ - {\text{(aq)}}\);
Allow only arrows as shown.
State symbols not needed.
Accept H2O and H3O+.
With HNO3:
faster rate of bubble/gas/hydrogen production;
faster rate of magnesium dissolving;
higher temperature change;
Accept opposite argument for HNO2.
Award [1] if 2 observations given but acid is not identified.
Reference to specific observations needed.
(i) (nitric acid) \({\text{7.5 c}}{{\text{m}}^{\text{3}}}\);
(ii) not valid as nitrous acid reacts with same volume/ \({\text{7.5 c}}{{\text{m}}^{\text{3}}}\);
HNO3;
(higher conductivity for solutions with same concentration as) there are more ions in solution;
change in oxidation numbers: Ag from 0 to +1 and N from +5 to +2;
Do not penalise missing charges on numbers.
balanced equation: \({\text{3Ag}} + {\text{NO}}_3^ - + {\text{4}}{{\text{H}}^ + } \to {\text{3A}}{{\text{g}}^ + } + {\text{NO}} + {\text{2}}{{\text{H}}_2}{\text{O}}\)
Award [1] for correct reactants and product;
Award [3] for correct balanced equation.
Ignore state symbols
Examiners report
This was the most popular question and it was well answered by the majority of candidates. The reaction was correctly described as exothermic and the reason for this explained correctly in most cases. Most candidates knew that yield would increase with increased pressure, but failed to score a second mark because they did not mention ‘gaseous’ although they did know the answer. The effect of increased temperature on rate was generally well described although some did get confused with yield and how it would affect equilibrium.
Most candidates correctly defined oxidation in 6(b)(i) but ‘hedged their bets’ by stating loss of electrons as well as an increase in oxidation number. In 6(b)(ii) the oxidation states were generally deduced correctly but sometimes written as ionic charges (5+ for instance, instead of +5).
In 6(c) most correctly defined strong and weak acids, and many also wrote correct equations. A few, though, had no idea. In (c), arrows proved to be a minefield for several candidates, especially the equilibrium sign. HA was commonly given, as were CH3COOH and HCl, instead of nitric and nitrous acid.
6(d) presented problems with many candidates unable to describe observations and instead stating there would be ‘more hydrogen produced’ or just that ‘the reaction would be faster’. However, better candidates were able to answer this part correctly and scored full marks.
In 6(e)(i) the calculation was answered well, but 6(e)(ii), that asked for a comment on the hypothesis, was not and few candidates stated that the same volume of acid was needed.
In 6(f), the majority correctly identified the strong acid but often failed to explain its better conductivity in terms of the ions.
Many could give a correct balanced equation and scored the 3 marks, and others scored 1 mark for giving the correct reactants and products. However, not many candidates used oxidation numbers to deduce the balanced equation.
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
A voltaic cell is made from a half-cell containing a magnesium electrode in a solution of magnesium nitrate and a half-cell containing a silver electrode in a solution of silver(I) nitrate.
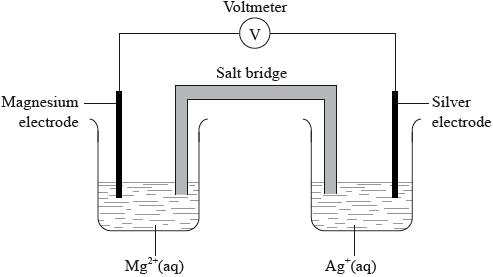
Hydrogen peroxide decomposes according to the equation below.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.

(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.

(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
(i) Given that magnesium is more reactive than silver, deduce the half-equations for the reactions occurring at each electrode, including state symbols.
Negative electrode (anode):
Positive electrode (cathode):
(ii) Outline one function of the salt bridge.
(i) State the property that determines the order in which elements are arranged in the periodic table.
(ii) State the relationship between the electron arrangement of an element and its group and period in the periodic table.
(i) The experiment is repeated with the same amount of a more effective catalyst, \({\text{Mn}}{{\text{O}}_{\text{2}}}\), under the same conditions and using the same concentration and volume of hydrogen peroxide. On the graph above, sketch the curve you would expect.
(ii) Outline how the initial rate of reaction can be found from the graph.
(iii) Outline a different experimental procedure that can be used to monitor the decomposition rate of hydrogen peroxide.
(iv) A Maxwell–Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.

Markscheme
(i) oxidation and (iron/Fe) loses electrons/increases in oxidation number/state;
(ii)  ;
;
Award [2] for five correct.
Award [1] for four correct.
Accept use of oxidation states (0, +1, –2, –2, +1) for oxidation numbers.
Penalize once for incorrect notation (eg, 2, 2–).
(iii) \({{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2Fe(s)}} \to {\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\);
Ignore state symbols.
(iv) Fe/iron;
oxygen is non-polar;
needs to break strong hydrogen bonds/H–bonds between water molecules (to dissolve) / oxygen cannot form hydrogen bonds/H–bonds with water;
oxygen can only form (weak) van der Waals’/vdW/LDF/London/dispersion forces with water;
(i) Negative electrode (anode):
\({\text{Mg(s)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }/\frac{1}{2}{\text{Mg(s)}} \to \frac{1}{2}{\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {{\text{e}}^ - }/\)
\({\text{Mg(s)}} - {\text{2}}{{\text{e}}^ - } \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}}/\frac{1}{2}{\text{Mg(s)}} - {{\text{e}}^ - } \to \frac{1}{2}{\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}}\);
Accept equations for the oxidation of water/hydroxide ions.
Positive electrode (cathode):
\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to {\text{Ag (s)}}\);
Accept Ag equation doubled so that both electrodes involve 2 electrons.
Accept e instead of e–.
Award [1 max] if both equations are correct but the state symbols are missing/incorrect.
Award [1 max] if both equations are reversed but state symbols correct.
(ii) provides ions that flow into electrolytes/half-cells / maintains electrical neutrality of solutions/electrolytes / provides electrical continuity by providing path for migrating ions;
Accept completes the (electrical) circuit / allows current to flow / OWTTE.
(i) atomic number / number of protons;
Accept number of electrons in a (neutral) atom.
(ii) groups indicate the number of electrons in the highest energy level/outer/valence shell;
periods indicate the number of (occupied) energy levels/shells (in the atom);
(i) steeper curve with a similar shape that reaches same maximum volume of \({{\text{O}}_{\text{2}}}\);
(ii) (draw a) tangent to the curve at origin/time = 0/start of reaction;
(calculate) the gradient/slope (of the tangent);
(iii) measure/monitor mass/pressure/\({\text{[}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{]}}\);
Accept measure/monitor temperature of system.
(iv) y-axis: probability / fraction of molecules/particles / probability density
Allow “number of particles/molecules” on y-axis.
and
x-axis: (kinetic) energy;
Accept “speed/velocity” on x-axis.

correct relative position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
more/greater proportion of molecules/collisions have the lower/required/catalysed \({E_{\text{a}}}\) (and can react upon collision);
M3 can be scored by shading and annotating the graph.
Accept a greater number/proportion of successful collisions as catalyst reduces Ea.
Examiners report
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
Group 7 of the periodic table contains a number of reactive elements such as chlorine, bromine and iodine.
Bleaches in which chlorine is the active ingredient are the most common, although some environmental groups have concerns about their use. In aqueous chlorine the equilibrium below produces chloric(I) acid (hypochlorous acid), HOCl, the active bleach.
\[{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HOCl (aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\]
Aqueous sodium chlorate(I), NaOCl, the most common active ingredient in chlorine based bleaches, oxidizes coloured materials to colourless products while being reduced to the chloride ion. It will also oxidize sulfur dioxide to the sulfate ion.
(i) Describe the colour change that occurs when aqueous chlorine is added to aqueous sodium bromide.
(ii) Outline, with the help of a chemical equation, why this reaction occurs.
The colour change in the reaction between aqueous chlorine and aqueous sodium iodide is very similar, but it differs with an excess of aqueous chlorine. Describe the appearance of the reaction mixture when excess aqueous chlorine has been added to aqueous sodium iodide.
Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is indicated in the equation above.
State a balanced equation for the reaction of chloric(I) acid with water.
Outline, in terms of the equilibrium above, why it is dangerous to use an acidic toilet cleaner in combination with this kind of bleach.
Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
Draw the Lewis (electron dot) structure of chloric(I) acid.
Predict the H–O–Cl bond angle in this molecule and explain this in terms of the valence shell electron pair repulsion (VSEPR) theory.
(i) Deduce the coefficients required to balance the half-equations given below.
___ \({\text{Cl}}{{\text{O}}^ - } + \) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({{\text{H}}_2}{\text{O}} + \) ___ \({\text{C}}{{\text{l}}^ - }\)
___ \({\text{SO}}_4^{2 - }\) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({\text{S}}{{\text{O}}_2} + \) ___ \({{\text{H}}_2}{\text{O}}\)
(ii) State the initial and final oxidation numbers of both chlorine and sulfur in the equations in part (i).

(iii) Use the half-equations to deduce the balanced equation for the reaction between the chlorate(I) ion and sulfur dioxide.
Markscheme
(i) from (pale) green/colourless to yellow/orange/brown;
Initial colour must be stated.
Do not accept “clear/transparent” instead of “colourless”.
(ii) chlorine more reactive/more powerful oxidizing agent (than bromine);
Accept opposite statements for bromine.
Accept “chloride ion a weaker reducing agent” / “bromide ion a stronger reducing agent”.
Accept “chlorine more electronegative than bromine”.
\({\text{C}}{{\text{l}}_2}{\text{(aq)}} + {\text{2NaBr(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2NaCl(aq)}}\) /
\({\text{C}}{{\text{l}}_2}{\text{(aq)}} + {\text{2B}}{{\text{r}}^ - }{\text{(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2C}}{{\text{l}}^ - }{\text{(aq)}}\);
Ignore state symbols.
Do not accept with equilibrium sign.
solid (in a colourless solution);
Accept “dark brown solution”.
chloric(I) acid (shown as) a molecule/molecular, but hydrochloric acid (shown as being) split into ions / OWTTE;
Accept “chloric(I) acid is partially dissociated and hydrochloric acid is fully dissociated”.
Reference needed to both acids for mark.
\({\text{HOCl(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}}/{\text{HOCl(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}}\);
Equilibrium sign required for the mark.
Ignore state symbols.
acid displaces the equilibrium to the left (to form chlorine);
chlorine is toxic/poisonous/harmful/lung irritant;
Accept answers that refer to the (c) (ii) equilibrium.
chloric(I) acid has –OH group / hydrogen attached to a very electronegative atom;
Accept polar molecule.
can form hydrogen bonds to water;
hydrogen bonding to water increases its solubility;
(as a weak acid it is) in equilibrium with ions;
 ;
;
Accept lines, dots or crosses to represent electron pairs.
\( \sim\)104°;
Accept values between 102° and 106°.
four electron pairs/regions of high electron density around O atom / electron pairs/regions of high electron density tetrahedrally arranged and two lone/non-bonding electron pairs on O atom;
Accept Lewis structure with two lone pairs on O and two angular bond pairs if given here as equivalent to M2.
lone pair–bonding pair repulsion greater than bonding pair–bonding pair repulsion;
(i) \({\text{(1) Cl}}{{\text{O}}^ - } + \) 2\(\,{{\text{H}}^ + } + \) 2\(\,{{\text{e}}^ - } \rightleftharpoons {\text{(1) }}{{\text{H}}_2}{\text{O}} + {\text{(1) C}}{{\text{l}}^ - }\);
\({\text{(1) SO}}_4^{2 - } + \) 4\(\,{{\text{H}}^ + } + \) 2\(\,{{\text{e}}^ - } \rightleftharpoons {\text{(1) S}}{{\text{O}}_2} + \) 2\(\,{{\text{H}}_2}{\text{O}}\);
(ii) Award [2] for all correct, [1] for 2 or 3 correct.

Remember to apply ECF from previous equations.
Penalize incorrect notation (eg, 4 or 4+ rather than +4) once only, so award [1] for a fully correct answer in an incorrect format.
(iii) \({\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} + {\text{S}}{{\text{O}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{SO}}_4^{2 - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\)
correct reactants and products;
balancing and cancelling \({{\text{e}}^ - }\), \({{\text{H}}^ + }\) and \({{\text{H}}_{\text{2}}}{\text{O}}\);
Ignore state symbols.
Do not penalize equilibrium sign.
Examiners report
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
The boiling points of the isomers of pentane, \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\), shown are 10, 28 and 36 °C, but not necessarily in that order.

Identify the boiling points for each of the isomers A, B and C and state a reason for your answer.

State the IUPAC names of isomers B and C.
B:
C:
Both \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) and \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\) can be used as fuels. Predict which compound would release a greater amount of heat per gram when it undergoes complete combustion. Suggest two reasons to support your prediction.
In many cities around the world, public transport vehicles use diesel, a liquid hydrocarbon fuel, which often contains sulfur impurities and undergoes incomplete combustion. All public transport vehicles in New Delhi, India, have been converted to use compressed natural gas (CNG) as fuel. Suggest two ways in which this improves air quality, giving a reason for your answer.
Markscheme

Award [1] if correct boiling points are assigned to 3 isomers.
increase in branching / more side chains / more spherical shape / reduced surface contact / less closely packed;
weaker intermolecular force/van der Waals’/London/dispersion forces;
Accept the opposite arguments
B: 2-methylbutane/methylbutane;
C: 2,2-dimethyl propane/dimethyl propane;
Do not penalize missing commas, hyphens or added spaces.
Do not accept 2-dimethylpropane, or 2,2-methylpropane.
\({{\text{C}}_5}{{\text{H}}_{12}}\);
Accept any two of the following explanations.
\({{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}}\) has greater molar mass / produces less grams of \({\text{C}}{{\text{O}}_2}\) and \({{\text{H}}_2}{\text{O}}\) per gram of the compound / suitable calculations to show this;
\({{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}}\) contains an O atom which contributes nothing to the energy released / partially oxidized / OWTTE;
analogous compounds such as butane and butan-1-ol show a lower value for the alcohol per mole in the data book / OWTTE;
the total bond strength in the pentanol molecule is higher than the total bond strength in pentane;
the total amount of energy produced in bond formation of the products per mole is the same;
fewer moles of pentanol in 1 g;
pentanol requires more energy to break intermolecular forces/hydrogen bonding / OWTTE;
Improvements [2]
less/no particulates/C/CO/VOC’s produced with CNG;
less/no SO2/SOx produced;
Reasons [1 max]
CO/SO2 toxic/poisonous;
SO2 causes acid rain;
CNG is likely to undergo complete/more combustion;
CNG has no/less sulfur impurities;
Examiners report
This question also featured on the G2 forms, as some teachers thought that the inclusion of Aim 8 type questions such as this would disadvantage candidates. However performance by the majority was very good. It should be noted that questions of this type will always be asked in future papers. In (a), most candidates correctly identified the boiling points although some reversed the order and a few had B with the highest boiling point. Explanations for this trend were not so well answered. Some candidates referred to breaking bonds in the carbon chain and several answers referred to the length of the carbon chain rather than the degree of branching.
The IUPAC names were generally well known, with the most common errors being the use of “pent” instead of “prop” and the omission of one of the locants, or “di” in “2,2-dimethylpropane”.
Many candidates scored 0 in part b) as they incorrectly suggested that pentan-1-ol would have a larger energy density than pentane. It is clear from the variety of wrong answers and reasons that candidates are not familiar with the ideas tested in this question. Many candidates referred to hydrogen bonds between molecules, as a reason for pentan-1-ol releasing more energy, only a few consulted their Data Booklet and made reference to this.
In c) there were 2 marks for improvements to air quality and 1 mark for a reason. Most candidates included the idea that there would be less carbon monoxide formed and that this was a poisonous gas. There were fewer references to oxides of sulfur, although many said that CNG has fewer S impurities rather than to say that less SO2/SOx is released, in this case as they had already scored their explanation mark they could not score for this and ended up with 2 marks out of 3. Some candidates did not centre their answer on what was being asked. Also, some candidates said that natural gas is a natural fuel while diesel is not, and that natural gas, when it burns does not produce carbon dioxide.
An acidic sample of a waste solution containing Sn2+(aq) reacted completely with K2Cr2O7 solution to form Sn4+(aq).
State the oxidation half-equation.
Deduce the overall redox equation for the reaction between acidic Sn2+(aq) and Cr2O72–(aq), using section 24 of the data booklet.
Calculate the percentage uncertainty for the mass of K2Cr2O7(s) from the given data.
The sample of K2Cr2O7(s) in (i) was dissolved in distilled water to form 0.100 dm3 solution. Calculate its molar concentration.
10.0 cm3 of the waste sample required 13.24 cm3 of the K2Cr2O7 solution. Calculate the molar concentration of Sn2+(aq) in the waste sample.
Markscheme
Sn2+(aq) → Sn4+(aq) + 2e–
Accept equilibrium sign.
Accept Sn2+(aq) – 2e– → Sn4+(aq).
[1 mark]
Cr2O72–(aq) + 14H+(aq) + 3Sn2+(aq) → 2Cr3+(aq) + 7H2O(l) + 3Sn4+(aq)
Accept equilibrium sign.
[1 mark]
«13.239 g ± 0.002 g so percentage uncertainty» 0.02 «%»
Accept answers given to greater precision, such as 0.0151%.
[1 mark]
« [K2Cr2O7] = \(\frac{{13.239{\text{ g}}}}{{294.20{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}} \times 0.100{\text{ d}}{{\text{m}}^3}}}\) =» 0.450 «mol\(\,\)dm–3»
[1 mark]
n(Sn2+) = «0.450 mol\(\,\)dm–3 x 0.01324 dm3 x \(\frac{{3\,mol}}{{1\,mol}}\) =» 0.0179 «mol»
«[Sn2+] = \(\frac{{0.0179\,mol}}{{0.0100\,mol}}\) =» 1.79 «mol\(\,\)dm–3»
Award [2] for correct final answer.
[2 marks]
Examiners report
Arsenic and nitrogen play a significant role in environmental chemistry. Arsenous acid, H3AsO3, can be found in oxygen-poor (anaerobic) water, and nitrogen-containing fertilizers can contaminate water.
Nitric acid, HNO3, is strong and nitrous acid, HNO2, is weak.
(i) Define oxidation and reduction in terms of electron loss or gain.
Oxidation:
Reduction:
(ii) Deduce the oxidation numbers of arsenic and nitrogen in each of the following species.
\({\text{A}}{{\text{s}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
\({\text{NO}}_3^ - \):
\({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{3}}}\):
\({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
(iii) Distinguish between the terms oxidizing agent and reducing agent.
(iv) In the removal of arsenic from contaminated groundwater, \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{3}}}\) is often first oxidized to arsenic acid, \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{4}}}\).
The following unbalanced redox reaction shows another method of forming \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{4}}}\).
\[{\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}} + {\text{NO}}_3^ - {\text{(aq)}} \to {{\text{H}}_3}{\text{As}}{{\text{O}}_4}{\text{(aq)}} + {{\text{N}}_2}{{\text{O}}_3}{\text{(aq)}}\]
Deduce the balanced redox equation in acid, and then identify both the oxidizing and reducing agents.
Define an acid according to the Brønsted–Lowry and Lewis theories.
Brønsted–Lowry theory:
Lewis theory:
The Lewis (electron dot) structure of nitrous acid is given below.

Identify which nitrogen-oxygen bond is the shorter.
Deduce the approximate value of the hydrogen-oxygen-nitrogen bond angle in nitrous acid and explain your answer.
Distinguish between a strong acid and a weak acid in terms of their dissociation in aqueous solution.
Ammonia, NH3, is a weak base. Deduce the Lewis (electron dot) structure of NH3. State the name of the shape of the molecule and explain why NH3 is a polar molecule.
When lime was added to a sample of soil, the pH changed from 5 to 7. Calculate the factor by which the hydrogen ion concentration changes.
One common nitrogen-containing fertilizer is ammonium sulfate. State its chemical formula.
Markscheme
(i) Oxidation: loss of electrons and Reduction: gain of electrons;
(ii) As2O3: +3;
NO3–: +5;
H3AsO3: +3;
N2O3: +3;
Penalize incorrect notation e.g. III, V, 3+, 5+, 3, 5 once only.
(iii) Oxidizing agent: substance reduced / removes electrons from another substance / causes some other substance to be oxidized / OWTTE and Reducing agent: substance oxidized / gives electrons to another substance / causes some other substance to be reduced / OWTTE;
Accept Oxidizing agent: electron/e/e– acceptor / causes oxidation / oxidation number/state decreases and Reducing agent: electron/e/e– donor / causes reduction / oxidation number/state increases.
(iv) \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}} + {\text{2NO}}_3^ - {\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {\text{2}}{{\text{H}}_3}{\text{As}}{{\text{O}}_4}{\text{(aq)}} + {{\text{N}}_2}{{\text{O}}_3}{\text{(aq)}}\)
correct coefficients for \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}\), \({{\text{H}}_3}{\text{As}}{{\text{O}}_4}\) and \({\text{NO}}_3^ - \), \({{\text{N}}_2}{{\text{O}}_3}\);
correct balanced equation;
Ignore state symbols.
M1 must be correct to award M2.
Oxidizing agent: \({\text{NO}}_3^ - {\text{(aq)}}\) / nitrate and Reducing agent: \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}}\) / arsenic(III) oxide;
Accept HNO3(aq)/nitric acid.
Accept arsenic oxide.
Species must be fully correct to score M3.
Ignore state symbols.
Brønsted Lowry theory: proton/H+ donor;
Lewis theory: electron-pair acceptor;
N=O;
accept any value in range 102–105°;
Actual value is 102°.
lone/non-bonding pairs on oxygen occupy more space/repel more than bonding pairs hence decreasing the H–O–N bond angle (from 109.5° ) / OWTTE;
Strong acid: acid/electrolyte completely/100% dissociated/ionized in solution/water / OWTTE and Weak acid: acid/electrolyte partially dissociated/ionized in solution/water / OWTTE;
 ;
;
Accept any combination of lines, dots or crosses to represent electron pairs.
trigonal/triangular pyramidal;
Accept pyramidal (since SL).
Do not allow tetrahedral.
net dipole moment present in molecule / NH bond polarities do not cancel each other out / unsymmetrical distribution of charge /OWTTE;
Do not accept molecule has no symmetry hence polar.
changes by 102 /100;
Allow changes from 10–5 to 10–7.
\({{\text{(N}}{{\text{H}}_4}{\text{)}}_2}{\text{S}}{{\text{O}}_4}\);
Examiners report
This was the most popular question answered in Section B.
The definition of oxidation and reduction, deducing oxidation numbers (although some forgot the + sign) and distinguishing between an oxidizing and reducing agent was answered very well by a majority of the candidates. However, a surprising number of candidates were unable to balance the redox equation or identify the correct oxidizing and reducing agents in the given reaction.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
Suggest how the change of iodine concentration could be followed.
A student produced these results with [H+] = 0.15 mol\(\,\)dm−3. Propanone and acid were in excess and iodine was the limiting reagent.
Determine the relative rate of reaction when [H+] = 0.15 mol\(\,\)dm−3.

The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
State and explain the relationship between the rate of reaction and the concentration of acid.
Markscheme
use a colorimeter/monitor the change in colour
OR
take samples AND quench AND titrate «with thiosulfate»
Accept change in pH.
Accept change in conductivity.
Accept other suitable methods.
Method must imply “change”.
[1 mark]

best fit line
relative rate of reaction = «\(\frac{{ - \Delta y}}{{\Delta x}} = \frac{{ - \left( {0.43 - 0.80} \right)}}{{50}}\) =» 0.0074/7.4 x 10−3
Best fit line required for M1.
M2 is independent of M1.
Accept range from 0.0070 to 0.0080.
[2 marks]
Relationship:
rate of reaction is «directly» proportional to [H+]
OR
rate of reaction \(\alpha \) [H+]
Explanation:
more frequent collisions/more collisions per unit of time «at greater concentration»
Accept "doubling the concentration doubles the rate".
Do not accept “rate increases as concentration increases”.
Do not accept collisions more likely.
[2 marks]
Examiners report
The diagram shows an incomplete voltaic cell with a light bulb in the circuit.
Identify the missing component of the cell and its function.
Deduce the half-equations for the reaction at each electrode when current flows.
Annotate the diagram with the location and direction of electron movement when current flows.
Markscheme
salt bridge
movement of ions
OR
balance charge
Do not accept “to complete circuit” unless ion movement is mentioned for M2.
[2 marks]
Positive electrode (cathode):
Ag+(aq) + e– → Ag(s)
Negative electrode (anode):
Mg(s) → Mg2+(aq) + 2e–
Award [1 max] if correct equations given at wrong electrodes.
[2 marks]
in external wire from left to right
[1 mark]
Examiners report
The emission spectrum of an element can be used to identify it.
Elements show trends in their physical properties across the periodic table.
Draw the first four energy levels of a hydrogen atom on the axis, labelling n = 1, 2, 3 and 4.
Draw the lines, on your diagram, that represent the electron transitions to n = 2 in the emission spectrum.
Outline why atomic radius decreases across period 3, sodium to chlorine.
Outline why the ionic radius of K+ is smaller than that of Cl−.
Copper is widely used as an electrical conductor.
Draw arrows in the boxes to represent the electronic configuration of copper in the 4s and 3d orbitals.
Impure copper can be purified by electrolysis. In the electrolytic cell, impure copper is the anode (positive electrode), pure copper is the cathode (negative electrode) and the electrolyte is copper(II) sulfate solution.
Formulate the half-equation at each electrode.
Outline where and in which direction the electrons flow during electrolysis.
Markscheme

4 levels showing convergence at higher energy
[1 mark]

arrows (pointing down) from n = 3 to n = 2 AND n = 4 to n = 2
[1 mark]
same number of shells/«outer» energy level/shielding AND nuclear charge/number of protons/Zeff increases «causing a stronger pull on the outer electrons»
[1 mark]
K+ 19 protons AND Cl– 17 protons
OR
K+ has «two» more protons
same number of electrons/isoelectronic «thus pulled closer together»
[2 marks]
[1 mark]
Anode (positive electrode):
Cu(s) → Cu2+(aq) + 2e–
Cathode (negative electrode):
Cu2+(aq) + 2e– → Cu(s)
Accept Cu(s) – 2e– → Cu2+(aq).
Accept \( \rightleftharpoons \) for →
Award [1 max] if the equations are at the wrong electrodes.
[2 marks]
«external» circuit/wire AND from positive/anode to negative/cathode electrode
Accept “through power supply/battery” instead of “circuit”.
[1 mark]
Examiners report
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
State the nuclear symbol notation, \({}_Z^AX\), for magnesium-26.
Mass spectroscopic analysis of a sample of magnesium gave the following results:
Calculate the relative atomic mass, Ar, of this sample of magnesium to two decimal places.
Magnesium burns in air to form a white compound, magnesium oxide. Formulate an equation for the reaction of magnesium oxide with water.
Describe the trend in acid-base properties of the oxides of period 3, sodium to chlorine.
In addition to magnesium oxide, magnesium forms another compound when burned in air. Suggest the formula of this compound
Describe the structure and bonding in solid magnesium oxide.
Magnesium chloride can be electrolysed.
Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922 K and 987 K respectively.
Anode (positive electrode):
Cathode (negative electrode):
Markscheme
\({}_{12}^{26}{\rm{Mg}}\)
«Ar =»\(\frac{{24 \times 78.60 + 25 \times 10.11 + 26 \times 11.29}}{{100}}\)
«= 24.3269 =» 24.33
Award [2] for correct final answer.
Do not accept data booklet value (24.31).
MgO(s) + H2O(l) → Mg(OH)2(s)
OR
MgO(s) + H2O(l) → Mg2+(aq) + 2OH–(aq)
Accept \( \rightleftharpoons \).
from basic to acidic
through amphoteric
Accept “alkali/alkaline” for “basic”.
Accept “oxides of Na and Mg: basic AND oxide of Al: amphoteric” for M1.
Accept “oxides of non-metals/Si to Cl acidic” for M2.
Do not accept just “become more acidic”
Mg3N2
Accept MgO2, Mg(OH)2, Mg(NOx)2, MgCO3.
«3-D/giant» regularly repeating arrangement «of ions»
OR
lattice «of ions»
Accept “giant” for M1, unless “giant covalent” stated.
electrostatic attraction between oppositely charged ions
OR
electrostatic attraction between Mg2+ and O2– ions
Do not accept “ionic” without description.
Anode (positive electrode):
2Cl– → Cl2(g) + 2e–
Cathode (negative electrode):
Mg2+ + 2e– → Mg(l)
Penalize missing/incorrect state symbols at Cl2 and Mg once only.
Award [1 max] if equations are at wrong electrodes.
Accept Mg (g).
Examiners report
Trends in physical and chemical properties are useful to chemists.
The Activity series lists the metal in order of reactivity.
Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
Describe the emission spectrum of hydrogen.
Identify the strongest reducing agent in the given list.
A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell.
Deduce the equation for the cell reaction.
The voltaic cell stated in part (ii) is partially shown below.
Draw and label the connections needed to show the direction of electron movement and ion flow between the two half-cells.
Markscheme
increasing number of protons
OR
increasing nuclear charge
«atomic» radius/size decreases
OR
same number of shells
OR
similar shielding «by inner electrons»
«greater energy needed to overcome increased attraction between nucleus and electrons»
atomic/ionic radius increases
smaller charge density
OR
force of attraction between metal ions and delocalised electrons decreases
Do not accept discussion of attraction between valence electrons and
nucleus for M2.
Accept “weaker metallic bonds” for M2.
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
Accept “P4O10 (s) + 2H2O (l) → 4HPO3 (aq)” (initial reaction).
«series of» lines
OR
only certain frequencies/wavelengths
convergence at high«er» frequency/energy/short«er» wavelength
M1 and/or M2 may be shown on a diagram.
Mn
Mn (s) + Ni2+ (aq) → Ni (s) + Mn2+ (aq)
wire between electrodes AND labelled salt bridge in contact with both electrolytes
anions to right (salt bridge)
OR
cations to left (salt bridge)
OR
arrow from Mn to Ni (on wire or next to it)
Electrodes can be connected directly or through voltmeter/ammeter/light bulb, but not a battery/power supply.
Accept ions or a specific salt as the label of the salt bridge.